KTP laser surgery offers a new way of selectively targeting microvasculature within laryngeal lesions and leaving normal surrounding tissues like epithelium and lamina propria intact – and thus preserving physiological phonation. This kind of selective photoangiolysis can be performed in awake patients in the office. With the introduction of KTP lasers we have a superb phonosurgical tool to address many laryngeal lesions and reduce iatrogenic induced scars, which likely would have occured with other phonosurgical techniques.
Introduction
Surgery is cutting. That is the simplest way to explain how surgeons approach tissues. After cutting the epithelium, surgeons can vary their ways of intervention, e.g. they can dissect, excise, coagulate, suture etc. However, and this is the bad news when it comes to vocal folds and postoperative voice production: not only do most lesions themselves stiffen the vocal fold and impede phonatory vibration, but also healing after cutting means inevitably inducing scar formation – and scars are always stiff. So, by just making ‘surgical’ interventions by cutting, on the one hand we hopefully can get rid of lesion-induced stiffness (and the lesion itself, of course), but on the other hand we put the pliability and softness of both structures, of the epithelium and of the lamina propria, at high risk for iatrogenic scar-induced stiffness. Always. Even worse: once scar-induced stiffness is present, there is hardly any scar softening intervention. But how can we target vocal fold lesions, epithelial or subepithelial, without surgically touching the tissues? This is where the KTP laser comes in.
Properties of the KTP laser
When ENT surgeons talk about laser surgery, they usually mean CO2 lasers. This laser targets water at 10,600 nm, and water is almost everywhere in the body. Depending on the energy absorbed, the effect can be coagulation, carbonisation, vapourisation or even ablation. But always the surface will be altered or even destroyed.
The KTP laser works differently. KTP (potassium titanyl phospate) targets red and brown chromophores at 532nm wavelength, and this essentially means blood. It is a ‘lucky circumstance’ that water does not absorb at that frequency. This allows for deep tissue penetration of the KTP in white, non-vascular tissue. Very simply put, the KTP goes through the vocal fold until it reaches a blood vessel. Here is one of the facts that makes this feature and the difference between CO2 and KTP very clear: red chromophores (such as oxyhemoglobin) absorb KTP approximately one million times more than white chromophores (such as white vocal folds). Translated into clinical practice, this means that – with appropriate energy levels – a KTP laser pulsed onto a white vocal fold with small superficial or subepithelial blood capillaries can result in selective coagulation of the blood within the vessel, leaving the surrounding unaltered, although the focussing beam was unselectively aimed at an area including the vessel and its white surrounding tissues. This effect is called selective photoangiolysis. With a CO2 laser or diode laser, all structures would have been at least unselectively coagulated or carbonised.
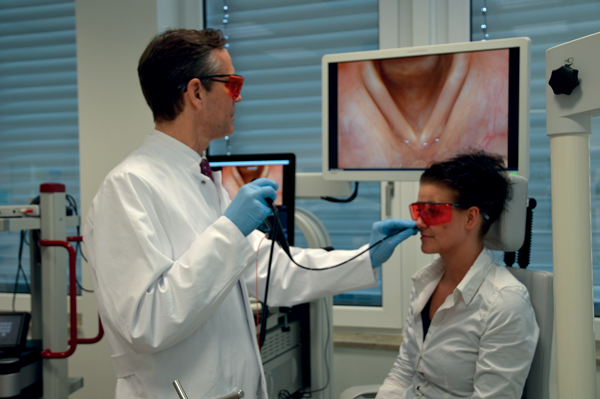
Figure 1: In-office transnasal laser surgery.
KTP laser fibre is routed through instrument channel.
Patient and surgeon wear protective glasses.
Phonosurgical in-office procedures
Beam delivery approaches
One great advantage in KTP laser surgery is the possibility to deliver the KTP laser beam to the target via glass fibres. Usually, the glass fibres are coated (with a so called cladding). The cladding protects the glass fibre and stabilises it at the same time. Fibres can be more than 2m long, enabling the placement of the laser machine apart from the endoscopy unit. Glass fibres have to be checked for cracks and failure before surgery starts. Also, the tip has to be prepared (and sometimes cut) so that the target beam is round without scattered radiation. It takes some practice to cut the glass fibre appropriately.
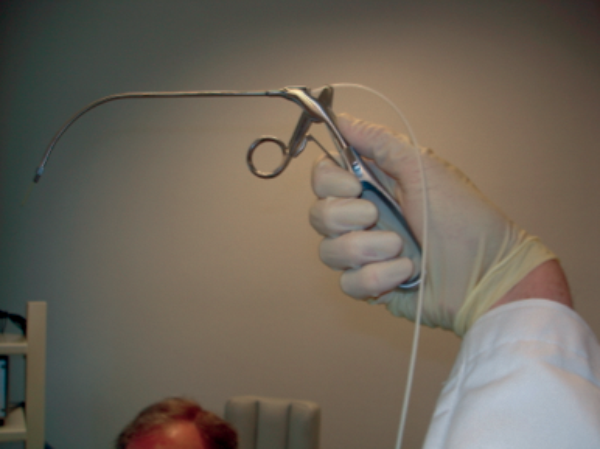
Figure 2: Bent cannula for routing KTP laser glass fibre into the larynx in indirect transoral laser surgery.
With our routine 400µm fibre we can perform most interventions with a transnasal fibreoptic approach using an Olympus ENF-VT2 tip chip endoscope with a 2mm instrument channel. Indirect transoral fibre routing can be performed by using a curved cannula held with the dominant hand while a rigid 70° endoscope monitors the endolaryngeal fibre position. The third approach is unusual, but may be an option in patients with pronounced gag responses. A 20G 70mm cannula can be routed percutaneously, as in percutaneous augmentation procedures, and the thyro-hyoid approach might be preferred since the glass fibre can be directed from a top-down direction onto the vocal folds.
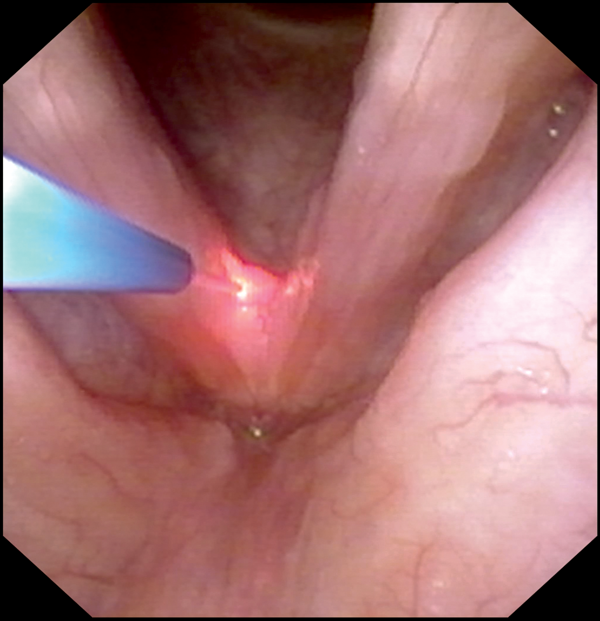
Figure A.
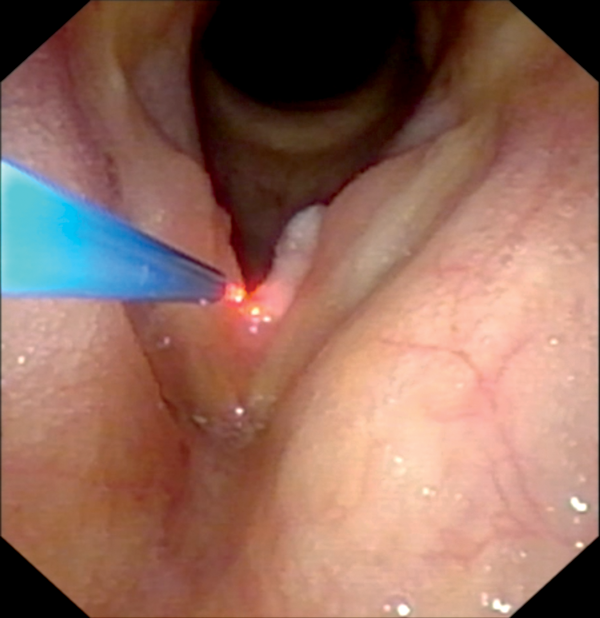
Figure B.
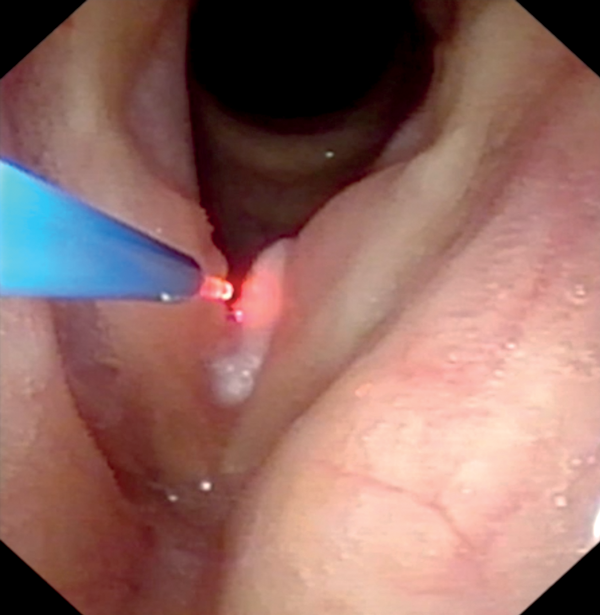
Figure C.
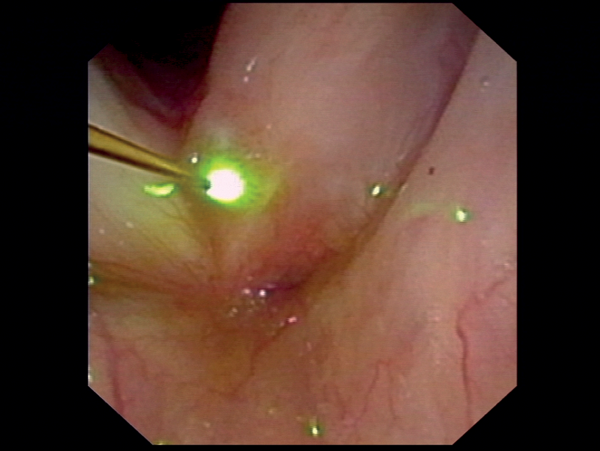
Figure D.
Figure 3: Transnasal fibreoscopy and lasering of papilloma (Figure A) with KTP laser at anterior commissure, (Figures B, C) with KTP laser at left vocal fold, (Figure D) with diode laser at left anterior vocal fold.
Contact and non-contact laser application
Laser energy can be applied with a continuous mode, or pulsed. KTP laser surgeons mostly prefer high energy levels in short pulses, so that the thermal relaxation time between pulses enables cooling of the lasered tissues. We apply maximum energy (8W, Nuvolas, ARC Laser Company, Nuremberg, Germany) and set the laser at 50-100 ms duty cycle and 300 ms off-cycle, and shorter pulses for ectatic vessels. When other KTP lasers offer higher energy levels, we would prefer higher intensity and shorter duty cycles. In most cases, the non-contact mode is used. However, when bleeding occurs or when local coagulation of tissues is needed, the contact mode leads to carbonisation of the glass fibre tip, resulting in its immediate heating. Thus, touching of tissue with the heated tip will coagulate or even carbonise the tissue. In this mode, the fibre tip acts like a ‘hot needle’. Unfortunately, the tip would have to be cleaned for subsequent non-contact lasering, which means an interruption of the surgical procedure.
Equipment and handling
Setting
The KTP laser is placed next to the endoscopy unit slightly behind the surgeon, and is controlled by a nurse. The laser pulses are activated by the surgeon via foot pedal. In transnasal interventions, the surgeon advances the flexible endoscope transnasally after topical anesthetisation of the upper airway with lidocaine 4%. Additional dripping of 1ml lidocaine 4% into the endolarynx through the instrument channel helps to suppress gag responses.
Safety
KTP laser beams are quite hazardous – and it would be very dangerous to believe that corrective eyeglasses and CO2 protective glasses protect our eyes from KTP laser injury. KTP laser beams (and their reflections) travel through white glass, window panes, microscopes and glass fibre endoscopes almost without any energy loss. When KTP laser beams are visible, the laser light travels through the eye until it reaches and destroys the retina. CO2 laser energy would have been absorbed at the cornea, due to its water content. Viewing of video camera recorded laser interventions on a monitor is not harming the eyes and does not necessitate safety glasses. In short, KTP laser interventions always necessitate KTP specific safety glasses (they have an orange colour).
Surgical interventions
Recurrent respiratory papilloma (RRP)
Treatment of RRP is a thankful indication for KTP laser surgery. Papilloma have capillary loops that respond well to pulsed noncontact laser coagulation – selective photoangiolysis. It is a tremendous advantage that KTP laser beams can penetrate several millimetres deep into non-vascular tissue and are able to selectively coagulate feeding vessels. This results in delayed fall-off of nonvital, involuted cells. We use a staggered glass fibrer advancement approach to get closer and closer to the papilloma surface, without touching it, while pulsing the KTP laser. Blanching of the surface mostly indicates that enough energy was delivered. Blanching is a combination of (superficial and deep) vessel photoangiolysis as well as a soft epithelial coagulation. KTP lasering is our first choice for treatment of papilloma within the anterior commissure because of its low risk of web formation. Of course, papilloma can also be coagulated in contact mode. The effect would resemble cutting lasers or radiofrequency heating with a needle.
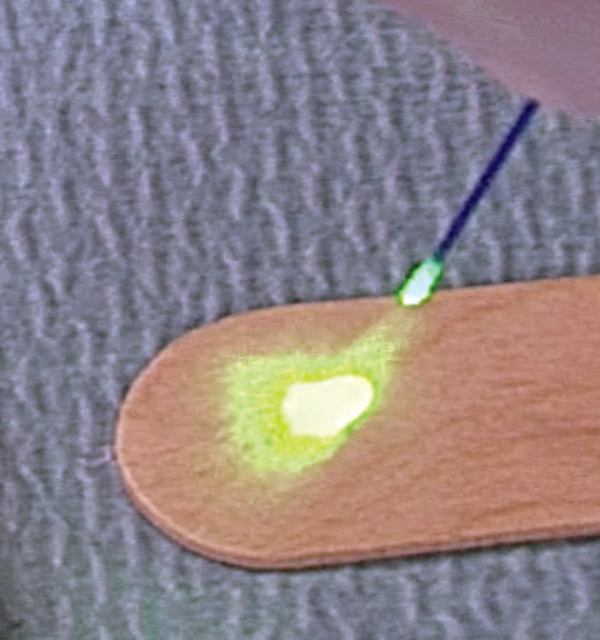
Figure 4A: Tip of fibre broken. Scattered pilot light beam.
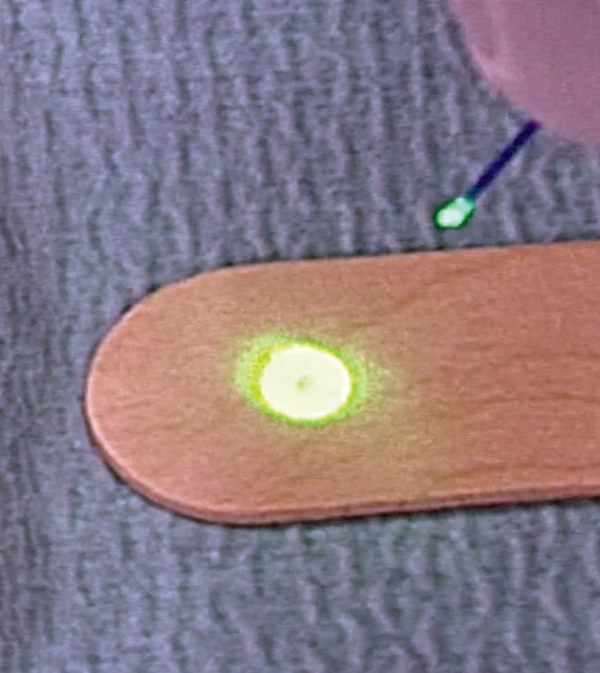
Figure 4B: Tip of fibre after adequate cutting. Pilot light beam is now rounded and ready for use.

Figure 5: KTP pulses on spectrally coloured paper (with hand piece, equal distances between pulse applications on paper along the spectrum, each pulse with 8 W, 100 ms duty cycle). Notice that yellow colour does not pick up energy, green only a little bit. Red and blue areas are carbonised.

Figure 6: Blood strands within egg white in a glass test tube. KTP laser travels through glass test tube and egg white. All energy absorbed at blood, which coagulates immediately.
Reinke’s oedema
Although Reinke’s oedema mostly looks more whitish-brownish and not red, it responds very well to KTP laser surgery with delayed involution. The reduction of oedema will be seen within the following weeks. But during the operation there remains this important question: When is enough laser energy applied? We look for superficial blanching as a visual marker. Surgeons and patients must get used to the fact that no immediate postsurgical voice effect occurs due to delayed lesion regression. Additional interventions may be needed.
Polyps
Similar to Reinke’s oedema, polyps also seem to respond to KTP laser surgery. Depending on the size of the polyp and its blood content, reactions will differ. Very vascular polyps would only coagulate on the surface, because blood shields the KTP beams from getting into the deeper parts of the polyp. In contact mode, the heating of the fibre tip could enable deep coagulation. In short, although KTP lasering is definitely not our first choice in vocal fold polyp removal, it is at least an alternative in our surgical armamentarium.
Vascular lesions
Epithelial vessels and vessels within the lamina propria can be selectively targeted with KTP lasers. Ideal effects of vessel coagulation depend on energy settings, pulsed versus non-pulsed modes, thickness and distance of glass fibre to the lesion, as well as tissue layer location and diameter of the vessel. There is no gold standard energy setting for vascular lesions. We have also successfully used the KTP laser in the interval in cases of patients with recurrent epistaxis with prominent vessels at the locus Kiesselbachii.
Contact granuloma
Contact granuloma respond well to KTP laser surgery. In bigger granuloma formations, we use the contact mode to coagulate the tissue. Interventions can be combined with transnasal local steroid injections and botulinum toxin injections into the lateral cricoarytenoid muscle.
Other lesions
Clinical experience revealed that leukoplakia and dysplastic lesions can be treated effectively with KTP laser. One of the major effects seems to be the photoangiolytic effect – a kind of ‘drying out’ of the lesions, and possible other tissue effects. Experience is needed for this indication, since blanching on white lesions cannot be used as visual indicator for energy deposition. We have also applied the KTP laser in cases of leukoplakia caused by biofilm formation – with successful long lasting effects.
Special issues
Hybrid laser technology
Until recently, each in-office laser procedure required its own laser fibre. With a recent hybrid laser technology, glass fibres delivering KTP laser beams at 532 nm can also deliver diode laser beams at 980 nm (Hybrid, ARC Laser) with different lasering features. Diode lasers at 980 nm penetrate deeper into the tissue and have a less selective photoangiolytic, but more overall tissue coagulation properties. The benefit of such a two-in-one technique (two different lasers feed one glass fibre) is that the surgeon can keep the endoscope with its laser glass fibre located at the surgical site and change from KTP to diode, or vice versa, with a simple switch. For example, deep tissue selective photoangiolysis can be started with KTP and, when a vessel ruptures and local bleeding occurs, the nonselective coagulative effect of a diode laser can be used for haemostasis. We prefer this hybrid option for, e.g., contact granuloma.
Mechanical use of the glass fibre
The stiffness of the 400µm glass fibre can be used to mechanically palpate, puncture, move and tear coagulated, blanched exophytic tissues during lasering pauses. It is a measure to remove and scrape off covering dead tissue in mass lesions, so that vital deeper layers can be uncovered, visualised, and laser interventions employed to continue debulking of lesions in haemostasis.
Suction of plume and secretions
With a 400µm glass fibre routed through the working channel of an endoscope, plume and secretions can be simultaneously suctioned during the in-office procedure. In cases of hypersalivation, excessive secretions can cover lesions and surgery cannot be continued. In patients with RRP, suction of plume is recommended to reduce viral spread with aerosols.
Conclusion
KTP laser surgery in the office offers an exciting new dimension of targeting microvasculature in many upper airway lesions. Appropriate use of KTP for phonosurgical interventions helps to protect the integrity of the layered structure of the vocal fold and its vibratory properties due to its feature of enabling selective photoangiolysis. High resolution in-office laryngoscopy and low complication rate with fibre-routed KTP laser makes this technique very appealing. We believe that KTP lasers should be added to the armamentarium of every phonosurgeon. Markus Hess, MD, ENT, phoniatrist, phonosurgeon,
Declaration of competing interests: None declared.