As technology improves, there are new ways to assist with surgeons when it comes to training and preparing for surgery. Barbara Anne Thomson and Georgios Kontorinis highlight how 3D printing can help with the understanding and surgical planning for complex skull base conditions.
Following the technological progress of medical devices, three-dimensional (3D) prints of various anatomical structures are increasingly being utilised in different fields of modern medicine [1,2]. Their impact on teaching and training, patients’ consultation and understanding, and surgical management has been evaluated in several studies showing promising data [1-4]. Particularly in specialties such as cardiothoracic surgery and neurosurgery, where the complexity of congenital malformations or anatomic variations can be challenging to understand for both the treatment team and the patients/parents, 3D models appear to be an additional tool [1-4].
Latest additions to the literature indicate the 3D prints to be very helpful during the consultation, enhancing engagement and communication and facilitating the understanding of the patient, as well as the relatives, of the extent and the location of the pathology and the challenge to access it minimising the collateral damage [1-4]. Furthermore, the usage of 3D prints for educational and training purposes is a rising field.
The utilisation of such technology in skull base surgery has only gradually been evolving over the last few years. Given the complexity of cases involving the skull base and the severe complications that can arise through operating in this anatomical area, utilising 3D prints can be a helpful tool for both the surgeon and the patient. Herein, we present the concept of 3D prints of the skull of patients with complex pathologies, and the potentials of such models in skull base surgery.
Technical description and clinical applications
Patients
We typically use 3D prints of the skull of patients with extensive skull base pathologies or subspecialised neurotological conditions. Informed consent is always obtained for ethical purposes. So far, we have used 3D prints in patients with giant jugular schwannomas undergoing staged extended transpetrous resections, with petrous apex chondrosarcoma, facial nerve schwannomas, superior semicircular canal dehiscence, and in patients following total rhinectomy, to better plan the implantation of the abutments for a prosthetic nose.
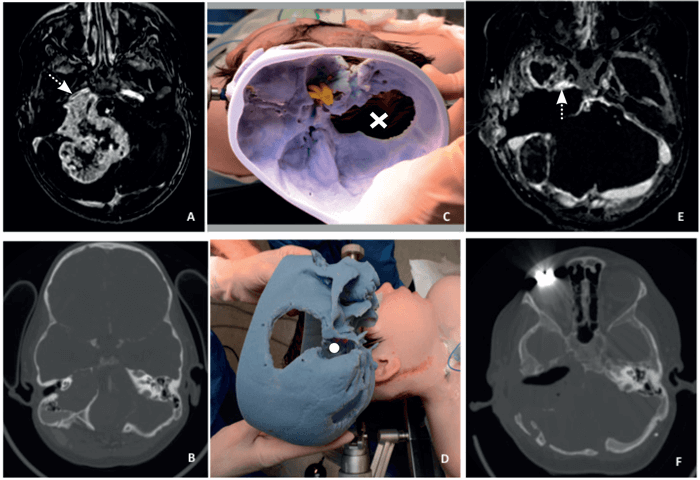
Figure 1. The preoperative MRI (A) and CT (B) of a patient with a right giant jugular schwannoma with immediate proximity to the right internal carotid artery (white arrow). We used the 3D-print intraoperatively (C and D); here shown in comparison with the patient’s positioning at the start of a staged procedure with part of the previous scar visible as well as the previous posterior craniotomy (X) and the cavity of the transpetrous approach (white dot) on the model. Finally, the MRI (E) and the CT (F) show the postoperative outcome with an extensive dissection and minimum residual on the internal carotid artery (dotted arrow).
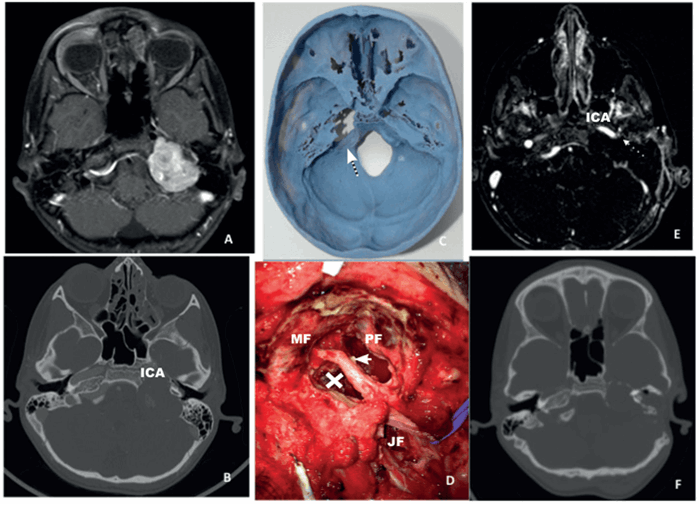
Figure 2. The preoperative MRI (A) and CT (B) of a patient with a left large jugular shwannoma showing the immediate proximity of the tumour to the internal carotid artery (ICA). The preoperatively obtained 3D print showing the precise position of the tumour (C) and a intraoperative picture (first stage procedure) showing the facial nerve (thick arrow) exiting the stylomastoid foramen, remnant of the tumour (X) on the ICA, the jugular foramen (JF), the middle fossa (MF) and the posterior fossa (PF). The final postoperative MRI (E) shows the final outcome with a small remnant of the capsule on the ICA while figure F shows an intermediate postoperative (after the first procedure) CT prior to the facial nerve transposition (case done in two stages).
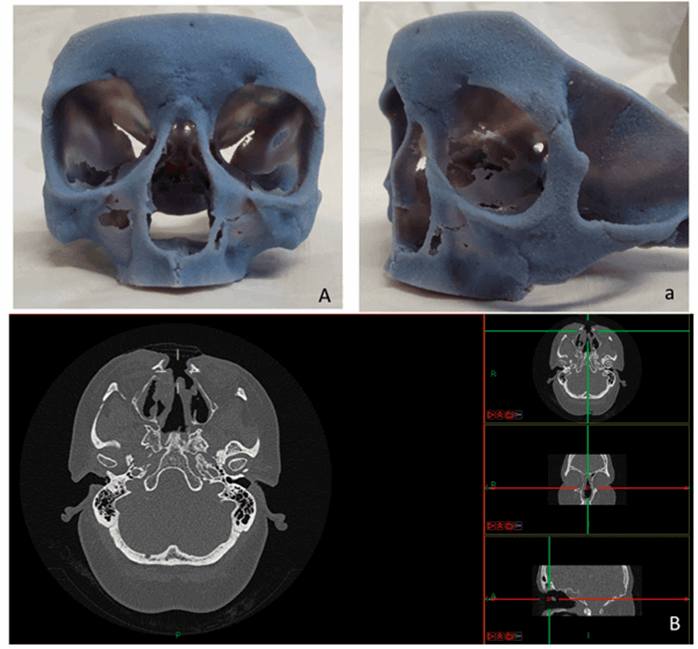
Figure 3. The 3D model of a patient following total rhinectomy and adjuvant radiotherapy prior the implantation of the abutments for the prosthesis (A/a) and the CT in three planes (B); the 3D print is utilised to find the areas with the thicker bone to safely accommodate the implant.
Three-dimensional prints
The 3D-prints (see Figures 1-3) are based on a high-resolution computed tomography (HRCT) scan of the temporal bone and the skull with a thickness of 0.5-0.6 mm (scans performed in various scanners in our department). The patient’s HRCT scan is exported into a 3D software package (Mimics, Materialise, Leuven, Belgium) to convert in to a digital imaging and communications in medicine (DICOM) file to which can be manipulated to reduce and target certain areas. The import of the DICOM file can easily and quickly create accurate 3D models. The software package allows the operator to anatomically analyse and virtually simulate surgery on screen. The final process involved exporting the 3D analysis and the printing of the 3D model. The stereolithographic file was exported to the 3D printer and the printing of model was finalised. We used the printer OBJET 30 (OBJECT 30, Stratasys, MN, United States) and rigid opaque photopolymers (Vero blue) as the printing material for our models; a material that allows detailed printing in blue colour (see Figures 1-3). The time needed for each 3D-model varied between 16 and 22 hours with an approximate cost of £340-£380 for every detailed model for an in-house production. Most of the time is associated with the actual printing, which is usually done overnight.
“The intraoperative use of the models is typically combined with navigation, when necessary, particularly in revision cases.”
Clinical application
We primarily use the models for perioperative planning (see Figures 1-3) but also during the consultations to better demonstrate to the patients and their relatives the extent of the disease, the structures involved (internal carotid artery, bulbar nerves, facial nerve, otic capsule, sigmoid sinus and the jugular bulb) and the planned approach. The intraoperative use of the models is typically combined with navigation, when necessary, particularly in revision cases. We also use the 3D prints for teaching and training purposes to explain to our trainees and medical students the complex anatomy of the skull base.
In cases of implantation of a prosthetic nose, the 3D print is used to identify the area with the thickest bone, where the implants can safely be placed. Considering that in cases of total rhinectomy due to malignancy, adjuvant radiotherapy will be used, resulting in poor bone quality, the identification of the most suitable areas for better placement seems extremely valuable. The software package can allow for measuring bone density and enabling surgical colleagues to correctly navigate and place implants in the correct vector.
“The software package can allow for measuring bone density and allowing surgical colleagues to correctly navigate and place implants in the correct vector.”
Discussion
Although the concept of 3D prints has been utilised in a few specialties, in skull base surgery it is still relatively unexplored, representing an emerging concept for teaching, training, planning and executing [2]. Such a concept comes with a very reasonable cost, considering the cost savings that a better-planned surgery has to offer. We found such models very useful for preoperative planning, particularly in revision or two-stage procedures, where the anatomic landmarks have been disrupted by both the pathology and the previous surgical intervention. Additionally, when it comes to a revision case, the skull base print can give, combined with navigation, a very accurate 3D understanding of the precise location of vital anatomical structures (see Figures 1 and 2).
A few studies assessing the impact of 3D models on surgical training reported promising data [4-6]. Such prints seem to offer an evolutionary method of understanding the anatomy of complex areas and the available approaches, and also facilitate medical teaching [4-6]. Generally, cadaveric dissection is considered the golden standard in surgical training. Lately, a direct comparison of cadaveric and 3D models showed such models to compare favourably to cadaveric skulls offering an additional training tool [6].
“A direct comparison of cadaveric and 3Dmodels showed such models to compare favourably to cadaveric skulls offering an additional training tool.”
As the technology evolves, 3D printers will become even more cost-efficient, allowing access to better material, able to separate tissue density (bone/soft tissue) and print in different colours. Access to such technology can be a powerful tool for the skull base surgeon, allowing careful perioperative planning and navigating, advanced teaching and training and more efficient clinician-patient communication.
References
1. Biglino G, Capelli C, Wray J, et al. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open 2015;5(4).
2. Crafts TD, Ellsperman SE, Wannemuehler TJ, et al. Three-Dimensional Printing and Its Applications in Otorhinolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg 2017;156:999-1010.
3. Pacione D, Tanweer O, Berman P, Harter DH. The utility of a multimaterial 3D printed model for surgical planning of complex deformity of the skull base and craniovertebral junction. J Neurosurg 2016;125:1194-7.
4. Ploch CC, Mansi CS, Jayamohan J, Kuhl E. Using 3D Printing to Create Personalized Brain Models for Neurosurgical Training and Preoperative Planning. World Neurosurg 2016;90:668-74.
5. Wanibuchi M, Noshiro S, Sugino T, et al. Training for Skull Base Surgery with a Colored Temporal Bone Model Created by Three-Dimensional Printing Technology. World Neurosurg 2016;91:66-72.
6. Hochman JB, Rhodes C, Wong D, et al. Comparison of cadaveric and isomorphic three-dimensional printed models in temporal bone education. Laryngoscope 2015;125:2353-7.
Declaration of Competing Interests: None declared.






