Professor Ad Snik has spent a large portion of his career in hearing implantation and has seen novel devices come and go, some of great benefit to patients, others which haven’t produced expected results. In this interview, he talks to Chris Coulson about a website he has recently developed to try and aid clinicians with their decisions regarding otological implantation.

We are really excited to hear about your new website to aid clinicians in choosing implantable hearing aids. Before we get on to the specifics, can you tell us a bit about your background?
First of all, thank you for giving me the opportunity to talk with you about my website. Concerning my background, I have a chair in audiology at the Radboud University in Nijmegen, the Netherlands. Just like all the other audiologists in the Netherlands, I have a master’s degree in physics, with an additional four years of training in audiology. Officially, I am a medical physicist / audiologist, and have been working as a clinical audiologist for more than 30 years.
I am employed at the ENT department of the Radboud University Medical Centre. The department is famous for its combined research in otology and audiology, while innovation is the key word. Concerning science, we work closely together with the department of Biophysics of our university, under the umbrella of the ‘Donders Centre for Neuroscience’. Our mutual project is called ‘Hearing and Implants’.
What type of auditory implants do you have experience with?
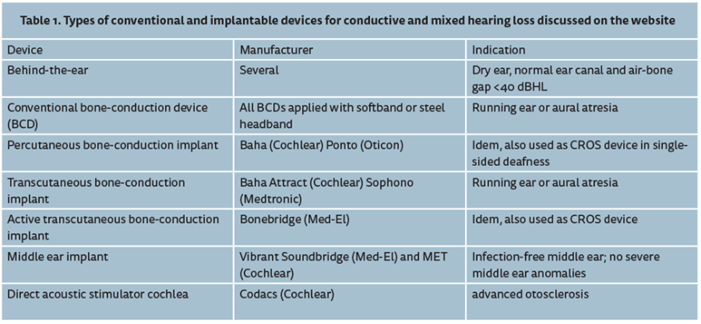
Currently, at our department we use cochlear implants and middle ear implants for sensorineural hearing loss. For patients with conductive and mixed hearing loss we apply various types of bone-conduction implants (either percutaneous or transcutaneous, active or passive) as well as middle ear implants with their actuator directly coupled to the cochlea. Table 1 presents an overview of the systems that we used.
Was there a specific event that made you realise there is a problem with implant companies’ claims about the effectiveness of their products?
The reason for me starting a website more than anything is me, getting older and approaching retirement. I felt the urge to summarise all our data in one easily accessible document, which was free of commercial interests and based as much as possible on objective data. As Ruth Bentler and her group have shown, answers to self-report questionnaires might easily be biased, especially when dealing with new technology (e.g. Bentler et al, 2003).
Companies might claim more than what is realistic. That is not unexpected because they have to run their business. It is our task as clinicians to put these claims into perspective. The website should be considered as an attempt to reach that goal. We have systematically studied the following devices with respect to audiological outcomes and clinical data-like stability.
Do regulatory bodies around the world have different standards for permitting implant use?
Probably, yes. Costs are high which might be a problem on its own in many countries. On the other hand, there are countries where, by law, optimal participation in the society is guaranteed for disabled inhabitants, with no clear financial restrictions. In other countries, hearing implants are reimbursed for patients for whom there is no acceptable alternative, thus as a last resort solution. Finally, in some counties, reimbursement depends on strict health-economical evaluations that should prove that the change in generic quality of life related to the extra costs of the treatment is below a certain limit.
What new method of assessing success of implants are you proposing?
First of all, it should be realised that the different types of implantable devices are not equivalent options at all; e.g. in terms of efficacy, stability, invasiveness and complexity of the surgery, MRI compatibility and costs. The first question we addressed is a simple one: how well does the implantable device work as an amplifier? Is it sufficiently powerful, with good amplification in the low and high frequencies, and is it more or less free of distortions? Table 2 shows an overview of one of our main outcome measures. This outcome measure is the maximum output or MPO, expressed in dB HL, which is the loudest sound that these implantable devices can produce. For technical details, visit the website; www.snikimplants.nl.
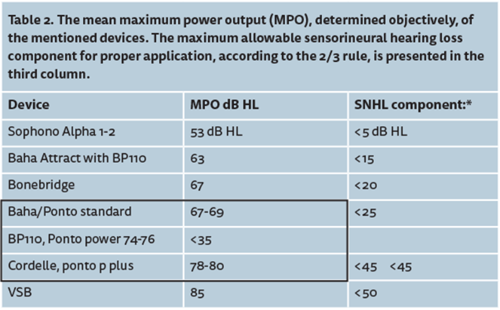
Table 2 shows that the loudest sounds that a device can produce (MPO) is limited and lies below loudness discomfort levels, which are typically found between 90 and 110dB HL. That means that the hearing range - the difference between the cochlear thresholds and loudness discomfort levels - will be limited by the MPO of the device; the upper part of the hearing range (loudness discomfort level – MPO) cannot be stimulated. That has consequences for proper application of these implantable devices.
Based on published data, a compromise was suggested concerning a ‘just-acceptable aided hearing range’, namely: a specific device for a given patient should only be used if the hearing range is at least 35dB (equals the width of the ‘speech banana’) while the unusable upper part of the hearing range is less than 1/3 of the total hearing range. This compromise is referred to as the 2/3 rule. Using this compromise, the implantable devices can now be categorised because the maximum allowable cochlear hearing loss component can be calculated (see Table 2, last column).
Next, the frequency response was evaluated, more specifically the effective gain at 0.5, 1, 2 and 4kHz. We performed a systematic review of literature, including all the implantable devices, for patients with conductive and mixed hearing loss. It was concluded that the gain was below expected values according to the NAL prescription rule (the NAL rule is the most frequently used prescription rule for gain and maximum output; see Dillon’s book, Hearing Aids). Probably, this was caused by a relatively low gain setting chosen by the patients to cope with the limited MPO of their device.
Only at 2kHz a reasonable match was found with NAL targets, not at the other frequencies, irrespective of the device used. Although all the technological improvements are impressive, there is an obvious need for devices with higher MPO and a broader frequency response to better rehabilitate patients with conductive and mixed hearing loss.
I have always wondered why we don’t have mandatory audit of success of all implantable devices (and indeed all operative results). If delivered on a worldwide basis, this would quickly assess the outcomes of a novel intervention. Are you aiming for the website to be a central repository database of all implanted data worldwide?
That is an important remark; I fully agree with you. My website is just a first step in the direction of more transparency, I hope. I guess we need to formulate a core set of outcome measures, as a first step to set up national and / or international databases, which in turn will enable us to evaluate issues like safety, stability and complication rates, efficacy, device use, patient’s satisfaction, etc. on a yearly basis. Furthermore, not only the pre and post intervention data should be collected but also long-term data. There are some initiatives in this direction but there are practical problems as it implies extra work and responsibility for clinicians, and managing the whole process requires a yearly budget.
Companies might not support such actions. They like to believe and advocate that their products are the best. They prefer to facilitate some implant centres to start up with a newly developed device and publish their data. However, such approaches have been criticised because it is not necessarily free of bias. Concerning the website, I would like to involve some established organisation that takes over or supports the website, for instance some international society of clinical professionals in our field.
Can anyone submit data to your website? And how will the information be treated?
Comments are most welcome. New data, if published, is also welcome. Subjective data like questionnaire results, commercial and anecdotal data will not be considered. Initially, we built in the option to respond directly via the website. Within the first five months, we received more than a hundred responses, all spam, so we have decided to skip that option. Email might be a more effective way to communicate. So, everyone is invited to comment via email.
How are you planning to publish the website to professionals and the public for the very few people who don’t read ENT and Audiology News?
Wow, I thought that I could stop my missionary work after this interview! I hope that your readers will spread this information to these few non-readers. Please note that the present website is not meant for the public, but rather for professionals in the field.
As a busy clinician, how do you take ‘time-out’ and relax?
I see my work not only as work but also as my hobby. For instance, developing this website has been quite relaxing for me. But don’t worry; my wife keeps me on a social and healthy track!
Interview conducted by Chris Coulson.
Declaration of Competing Interests: None declared.