The pandemic has driven innovation in ways that we have not seen for many decades. Intensive care medicine and ENT have been at the forefront of these advances, and our good friends David Howard (never one to put his feet up in retirement!) and Vik Veer have been involved in a collaboration that may prove to be one of the most exciting developments in medicine for many years.
It is a fact that natural disasters, wars and global pandemics not unusually bring about great acts of kindness and notable improvements in medicine, engineering, and other aspects of life. Unfortunately, it is also a sad reflection that these frequently take place in wealthy countries and not in the poorer countries of the world. Let us hope that the challenge of COVID-19 proves to be an exception over the next few years.
I have been fortunate to work within a group of amazing engineers and medics since their formation in March 2020. When the pandemic started to become a massive concern, the government put out details of the UK Ventilator Challenge to combat the perceived potential shortage of ventilators in ICUs around the UK. A young civil engineer, Dave McKeown, who normally works on flood defences for the UK Environment Agency, decided to give this considerable thought but, instead of designing a positive pressure ventilation system, he decided that a modern lightweight version of the negative pressure ‘iron lung’ from the 1930-1950s could be manufactured much more rapidly and at a much lower cost, for both wealthy and poorer countries. Negative pressure ventilators assist natural respiration by encouraging and amplifying the natural movements of the chest and respiratory muscles, rather than forcing air into the lungs by positive pressure.
Fortunately, Dave was totally unaware that a whole generation of anaesthetists, critical care and respiratory medicine doctors have grown up using only positive pressure devices and he, therefore, made this decision in a totally unbiased manner. He was also fortunate to receive the initial support of Dr Malcolm Coulthard, a semi-retired Consultant Paediatrician from Newcastle, whose love of physiology and innovation in medicine was, and still is, crucial. On 19 March, he wrote to the Cabinet Office asking for support with regard to his idea. He did not receive a reply and is still waiting!
However, a person, or persons, unknown shared the idea on a Facebook post, and I was contacted by a young foundation doctor who had seen the post, Aleksandra Kotwica, who I have worked with previously in the Xtreme-Everest Medical Research team at Everest Base Camp and in Tanzania, Africa. The conversation went along the lines that as I was now so old, I wouldn’t be able to do anything in the hospital and I would have little to do to do in lockdown! So, perhaps I might like to contact this crazy engineer to see if I could help. Later that day I joined a small, truly eclectic, group of engineers and doctors that formed the initial team that we called ‘Exovent’. There can be advantages to age; I remember as a medical student and young doctor in the 1960s and 70s seeing negative pressure ventilators still in use. The concept and physiological advantages were well known to me. I undertook one of the first intercalated BSc degrees in cardiovascular and respiratory physiology in 1968.
The Exovent team
Our first WhatsApp group was formed on 21 March 2020 and the aim was clear: to produce a working device in the shortest possible timescale. Our brilliant senior engineers, Nick Ryan (Steer Energy Solutions) and Neil Downie (and Neil’s incredible wife, Diane, retired GP/Physiologist/Engineer!) built working prototypes in their garages in just 24 hours, using large lightweight plastic boxes, plywood, material from a diving suit to form seals, vacuum cleaner motors for power and the simplest effective control system. Before we could prevent Nick, he was testing himself in the device with negative pressures down to -60 cm H2O! These prototypes proved that the concept worked and that, with simple modern materials, a powerful system could be rapidly produced to allow a person’s ventilation to be supported either by the simple application of a continuous negative extra-thoracic pressure (CNEP) and a very important concept, or additionally by the cyclical application of negative pressure ventilation (NPV), as was applied by the old iron lung devices.
“Fortunately, Dave was totally unaware that a whole generation of anaesthetists, critical care and respiratory medicine doctors have grown up using only positive pressure devices”
The ‘iron lungs’, first designed in 1928, had supported patients’ ventilation when they had been paralysed by the polio virus, in many cases for decades of their remaining life. There are multiple accounts and books written by these survivors, many of which are truly fascinating, which is why a whole generation of people and doctors relate negative pressure ventilation to polio in the first half of the 20th century. In contrast, the vast majority of people, including doctors, are completely unaware that negative pressure devices continued to be used and researched in many acute and chronic forms of ventilatory failure in children and adults (pneumonia, ARDS, COPD etc.,) to the present day.
The Exovent team rapidly expanded within just a few days with a multiplicity of telephone calls, late-night working, whole weekends devoted to the project and the institution of multiple and prolonged Zoom meetings which have now become so common in everyday life. I immediately co-opted my long-time consultant anaesthestist colleagues, Anil Patel and Jim Roberts from the Royal National Throat, Nose and Ear Hospital (RNTNE) and they in turn have brought in other wonderful innovative colleagues, including Peter Young, Critical Care Consultant, and Emily Hodges, Senior ICU Nurse, from Queen Elizabeth Hospital, Kings Lynn.
We now have over 50 members in the group, including colleagues covering many aspects of engineering, medical and surgical disciplines, including critical care, anaesthesia, respiratory medicine and virtually every aspect of commercial enabling, regulation, PR, marketing and manufacturing. It will take a very substantial book to describe the team and its intense activities since last March. It would also be invidious to start singling out colleagues in this short article, as any one of them could take up several pages. For those of you interested, take a few minutes to go onto www.exovent.org and read the short blogs written by some of the team members of those early days, like Jon Harris, Chief Engineer for Costa Coffee, who was released from his full-time job to assist us. Unfortunately, he couldn’t provide us with never-ending free coffee by Zoom in those early days, but his expertise in engineering and enabling was invaluable!
“Before we could prevent Nick, he was testing himself in the device with negative pressures down to -60 cm H2O!”
I was delighted to be joined by ENT consultants, Cath Rennie from Charing Cross and Vik Veer from the Royal National Throat, Nose and Ear Hospital at UCLH. Cath has been working closely with the Mercedes–Benz F1 team, developing ways to reduce aerosol spread in the hospital environment, but that is another whole story for ENT & Audiology News! Cath’s innovation and engineering skills have resulted in her receiving a prestigious recognition as one of the top 50 Women in Engineering 2021 (See our interview with Catherine Rennie following this award).
Vik has added greatly to our understanding of the potential for negative pressure use in chronic conditions and he is joining me in this article. Vik has superb communication skills, and his video contributions are thought provoking and highly instructive. I have asked him to include his experiences here with the Exovent team.
Vik Veer
Although Exovent was conceived in response to the COVID pandemic threat, the potential long-term benefits of this technology were evident from the outset. The ability to ventilate patients in acute respiratory distress (from a variety of causes), without necessarily residing in the intensive care unit, would be a great advantage beyond the COVID era. Additionally, one may envisage a ‘negative pressure ventilation ward’ where patients who no longer require one-to-one nursing support in the intensive care and high-dependence units may be allowed to convalesce in relative peace. This has the clear advantage of freeing up intensive care beds for those who truly need this level of care. Certain patients may benefit from avoiding a temporary tracheostomy that would otherwise be required for the purposes of ‘weaning’. The Exovent option may also reduce the complications of prolonged positive pressure endotracheal ventilation, such as glottic and tracheal stenosis, pneumothorax and other respiratory side-effects.
This hypothetical negative pressure ventilation ward may also become the postoperative ward of choice after certain procedures such as thoracic surgery, where there is a potentially higher risk of pneumothorax. Also consider tracheal, or laryngopharyngeal reconstructive surgery where the presence of an endotracheal tube within the operative space may be considered a disadvantage for wound healing.
Beyond the confines of hospital medicine, Exovent may benefit the sufferers of certain chronic diseases. Amyotrophic lateral sclerosis, neuromuscular dystrophy, myasthenia gravis and multiple sclerosis are examples of neuromuscular conditions where patients may be able to prolong the time where they can avoid requiring admission and dependence on hospital support.
My personal interests lie in how Exovent may benefit the lives of patients with sleep disorders. Central sleep apnoea is a relatively rare condition that is often poorly managed on continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), but a viable and comfortable alternative may be a boon to this tight-nit community. I see possibilities in patients with obesity hypoventilation syndrome, where extreme body weight precludes the ability to take adequate ventilatory breaths, particularly during sleep when respiratory drive is reduced. I also see morbidly obese patients at a particularly high risk from positive pressure ventilation due to the forces needed to achieve adequate ventilation. Although there are obstacles to overcome, I believe we also have some evidence that Exovent may be of benefit to certain obstructive sleep apnoea phenotypes, particularly those who struggle with CPAP. Even if the benefits are limited in the various patient classes described above, the plethora of sensors and real-time measurements that can be incorporated into the Exovent system would provide us with enough data to sate the hungriest of artificial minds.
“Although Exovent was conceived in response to the COVID pandemic threat, the potential long-term benefits of this technology were evident from the outset”
The collective experiences from engineering, medicine and life in general, are amazing. Of course, in a sense this is not surprising as they are self-selecting and their commitment to our humanitarian project comes as no surprise. We formed our UK charity, Exovent, in April 2020 in just 24 hours, which must be a record for the Charities Commission, thanks to Ian Joesbury, our tireless CEO and colleagues in the world of PR and banking.
Manufacturing and initial testing of the Exovent negative-pressure respiratory support device
The early period of March and April 2020 was a very worrying time for everyone as COVID-19 was proving to be different from previous acute infectious diseases and the best treatment for patients was not clear. There were great concerns with regard to the safety of all frontline health workers and it was particularly clear to us when those exhausted members joined the Zoom meetings. Archie McPherson, CEO with WMG Centre High-Value Manufacturing Catapult joined one of our Zoom meetings via Jan Patalong, a marketing maestro who had seen the Facebook post and kindly put the Exovent group in touch with Marshall ADG in Cambridge, a leading global Aerospace Company. A truly phenomenal period of intense work meant that six weeks later on 29 April, we tested the first lightweight (10kg) prototype unit at Marshall’s Aerospace factory.
Testing was performed on six healthy adult volunteer members of the development team, after full ergonomic bench-testing had been completed and in the presence of our three senior anaesthetists from Exovent (Figure 1).

Figure 1. Consultant Anaesthetist, Dr Jim Roberts, comfortably asleep
in the Exovent whilst undergoing negative pressure ventilation, NPV.
All subjects were studied while lying supine with the upper half of the bed and Exovent in a 30° head-up position, supine without elevation, and prone with a 10° head-up tilt. Tidal volume and vital capacity were measured at rest, then tidal volumes were measured after the application for two minutes of each of the following CNEP pressures: -5 cmH2O; -10 cmH2O; -15 cmH2O; and -20 cmH2O. These were delivered in a random order and with the subject blinded to the applied pressure. We then measured the tidal volumes generated during ventilation at four combinations of negative-pressure ventilation and negative extra-thoracic end-expiratory pressure (NEEP) settings. The combination of NPV and NEEP produced effective ventilation, with the resting tidal volume being exceeded by the application of just -4 cmH2O of extra-thoracic negative pressure. All the volunteers found the experience comfortable (I fell asleep for most of the time) and none had ventilator dyssynchrony. It is striking, looking at the video clips and photos of our human volunteer testing, how relaxed and comfortable the subjects were whilst in the Exovent, being able to move and alter their position and how they were able to talk quite easily during the expiratory phase (Figure 2).

Figure 2. David talking to the team whilst undergoing both CNEP and NPV testing.
This ability to communicate whilst being ventilated is a huge advantage. It was also possible to sip a drink via a straw and eat without difficulty (Figures 3-5).
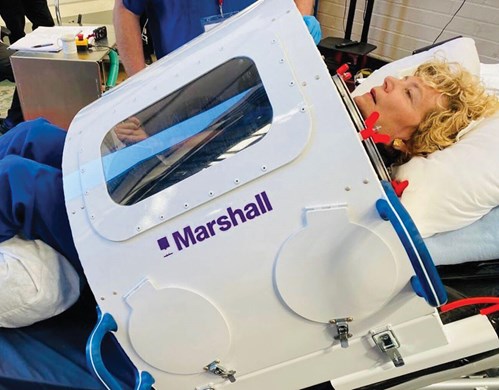
Figure 3. Regular readers of ENT & Audiology News will have seen Professor Valerie Lund
on many occasions but not like this! Here she is relaxing whilst undergoing cyclical NPV.
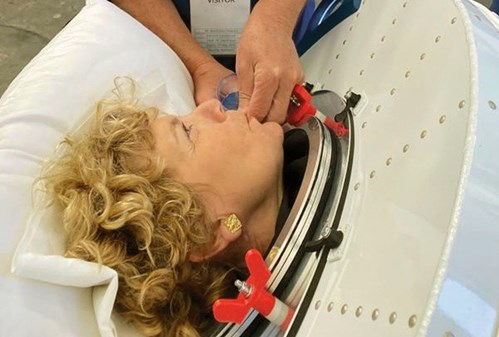
Figure 4. Valerie sipping a drink via a straw during ventilation.
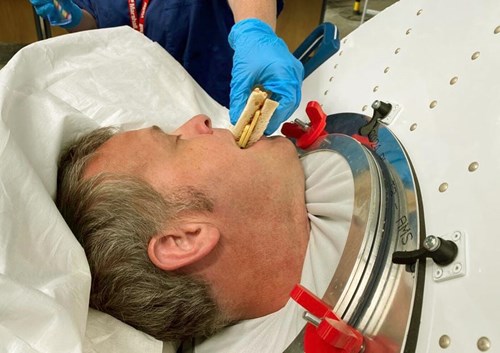
Figure 5. Dr Peter Young, Consultant in Critical Care and Anaesthesia,
eating a cheese sandwich during NPV.
Our design allowed good nursing and monitoring access (Figure 6).
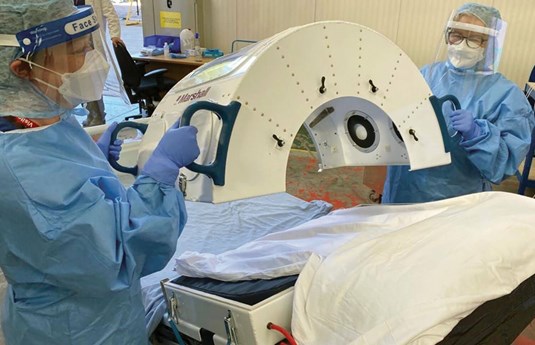
Figure 6. Dr Diane Downie and Sister Emily Hodges from Critical Care
testing the practicalities and ergonomics of Exovent.
These details were collated and written up by Dr Malcolm Coulthard and the Exovent team. Full details of this testing can be found in Anaesthesia [1].
Subsequently, we have been extremely fortunate to gain the support of the Southampton University Hospital Critical Care and Respiratory High Dependency Unit teams under the leadership of Professor Mike Grocott, Director of the Southampton NIHR Biomedical Research Centre, in addition to Dr Peter Young and the team at Queen Elizabeth Hospital, Kings Lynn. The clinical staff of both hospitals have been working out the best ways to use Exovent for the patients (Figure 8).
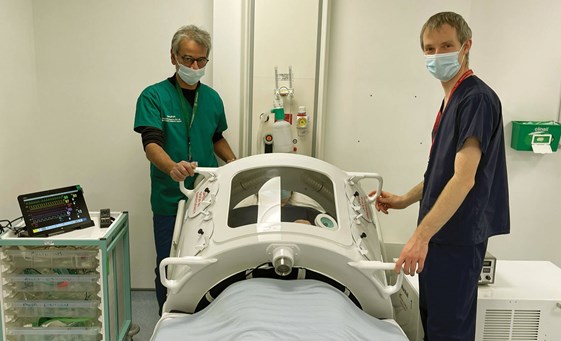
Figure 7. Professor John Pappachan, Consultant in Paediatric ICU, and Dr Andy Cumpsey,
Anaesthetic Fellow, carrying out further testing of the Exovent at Southampton University Hospital.
It is so important that the nursing and other staff evaluate even simple things, such as patient information, explanation of the device, monitoring, supplemental facemask oxygen, intravenous lines, cleaning, and all the everyday aspects of use. Mike Grocott kindly presented the Exovent team’s work to the first Innovation Network Forum of the Intensive Care Society of the UK on 20 May 2021.
As anyone knows who has tried to introduce a major innovative medical device, there are many trials and tribulations to work through. Our initial testing demonstrated the need for some improvements from the initial prototype and our wonderful new partners at Portsmouth Aviation have taken over the subsequent development and manufacture of the first adult Exovent device with the kind assistance of full information from Marshall ADG. Portsmouth Aviation are intent on becoming a medical device manufacturer as a new arm to their considerable engineering work. Full technical files and all necessary information are being prepared for MHRA and clinical trials will commence later this year at Southampton. Multiple grant applications and requests for financial support are ongoing and we are actively seeking financial investment.
“Exovent does not require to be driven by pressurised air or oxygen, markedly reducing the demands on oxygen supplies”
Product development continues with the design and production of a low-cost global device for low- and middle-income countries (LMICs), a specific paediatric device which we hope to develop in association with the Mercedes AMG F1 team. Our website and charity structure likewise are undergoing continuous development and we have achieved some wonderful international collaboration in Bangladesh, Ghana, Canada, Qatar and Taiwan. In particular, we must mention that the Bangladesh team have developed their own lightweight device made of composite material, and have already undertaken their initial clinical trials and submission to their health service and regulator. Their progress has been the most rapid of all, with assistance from the Exovent team and we are absolutely delighted with their progress. We are in touch with the Gates foundation, who are particularly interested in a paediatric device for LMICs. A low-cost device that is rapid to produce and simpler to use has huge potential globally.
Ironically, it was the shortage of negative pressure ‘iron lung-type’ devices in the polio epidemic in Copenhagen in 1952, which stimulated the development of positive pressure ventilation applied to tracheostomised and intubated patients. This development continued in the 1960s to the present day so that a whole generation of doctors have forgotten the advantages of negative pressure. We have learnt that positive pressure ventilation, whilst lifesaving for many patients, has untoward effects such as ventilator-assisted pneumonia, airway trauma, reduced cardiac output by compromise of venous return, pneumothorax, the need for paralysis and sedation with loss of communication and swallowing, and the potential for muscle loss and neuropathy. These potential disadvantages have been all too evident in some hospitalised patients during COVID-19.
Summary
A lightweight, torso-only, negative pressure device that is effective, easy to use and low cost to manufacture has enormous potential to reintroduce negative pressure respiratory support as an alternative to the sophisticated and often expensive modern positive pressure devices. Exovent can provide an alternative choice to using continuous positive airway pressure, by delivering continuous negative extra-thoracic pressure (CNEP). Exovent does not require to be driven by pressurised air or oxygen, markedly reducing the demands on oxygen supplies. This is an important advantage, even in wealthy countries, as COVID-19 has shown and particularly important in LMICs where oxygen is a major issue. Any additional oxygen that the patient requires is provided by facemask or nasal prongs. We look forward to continuing its development and will be publishing the results of our clinical trials.
References
1. The Exovent Development Group. Exovent: a study of a new negative-pressure ventilatory support device in healthy adults. Anaesthesia 2021;76(5):623-8.
'Spotlight on Innovation’ is an informative section to provide insight and discussion on recent advances in technology and research and does not imply endorsement by ENT & Audiology News.




