A new implant for bone-conduction hearing, BCI (Bone Conduction Implant), has been developed by Bo Håkansson and his team of researchers at Chalmers University of Technology and Sahlgrenska University Hospital, both in Gothenburg, Sweden. Unlike most bone-conduction devices used today, the new hearing implant does not need a titanium screw anchorage in the skull bone. The BCI transmits vibrations directly to the mastoid bone and the skin is kept intact (so called direct drive active transcutaneous system).
The BCI implant uses the proprietary BEST transducer (Balanced Electromagnetic Separation Transducer) that is less than half the size of transducers found in comparable products. In a recently published study, the research group found that the BCI implant would fit in 95% of patient mastoid bones based on CT images [1].
The BCI system consists of an external audio processor, and an implanted part called the Bridging Bone Conductor (BBC). With a simple surgical procedure the transducer part of the BBC is placed in a 5mm drilled recess just behind the pinna. The transducer has a flat contact to the mastoid bone and no screws are used. The audio processor is attached to the skin using slight magnetic force and can easily be attached, and removed. Sound signals are transmitted to the BBC with magnetic induction.
The transducer has a flat contact to the mastoid bone and no screws are used.
There are many advantages with the BCI system compared to the percutaneous bone anchored system:
- The skin is intact. Therefore fewer skin complications are expected, no maintenance of the skin is needed and the audio processor is easier to attach and to remove.
- No screw is used. The risk of losing a screw is eliminated.
- The position of the transducer is closer to the cochlea implying a more efficient sound transmission to the cochlea.
- The distance between the audio processor and the transducer is larger leading to less feed-back problems.
- The BEST transducer has a high frequency boost with higher amplification in the high frequency range which is important for speech perception.
Compared to bone-conduction systems where the transducer is placed on the skin (skin drive passive transcutaneous systems) the inherent dampening of high frequency sounds that comes with skin drive systems is avoided. Further, the force with which the audio processor is attached to the skin is less with the BCI compared to skin drive systems, implying less risk of skin problems due to obstructed blood flow in the tissues between the implanted magnet and the magnet in the audio processor.
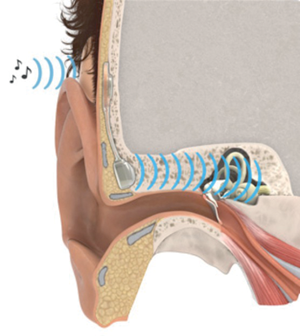
Bridging Bone Conductor (BBC).
The first operation was performed in December 2012 by Måns Eeg-Olofsson, Senior Surgeon at the ENT department at Sahlgrenska University Hospital, Gothenburg [2]. As part of an ongoing clinical trial of the new implant system, the interim data from the first six patients with a minimum of six months’ follow-up was reviewed by the Swedish competent authority, and the study was approved for a continuation at multiple centres. This first six month follow-up data has now been accepted for publication [3]. The report includes six patients (age 18-67 years) with mild-to-moderate conductive or mixed hearing loss. As a reference, all patients were tested using a state-of-the-art bone conduction device on a soft band (Ponto Pro Power from Oticon Medical).
Bo Håkansson (right), Chalmers University of Technology, and Måns Eeg-Olofsson (left),
ENT surgeon at Sahlgrenska University Hospital. Both in Gothenburg, Sweden.
The surgical procedure was found to be simple and uncomplicated with no adverse events. The improvement in aided thresholds compared to the unaided situation was as expected highly significant, as were all other audiological outcomes. These included speech recognition thresholds, speech recognition score in quiet and in noise at conversational levels, as well as validated quality of life questionnaires. All BCI results were better or equal to the reference power device results. The aided thresholds were generally higher with the BCI as compared with the reference power device, and reached significant differences up to 10dB in the mid-frequency range.
The research group concludes, based on these clinical results, that “BCI provides significant hearing rehabilitation for patients with mild-to-moderate conductive or mixed hearing impairments, and can be easily and safely implanted”.
In 2014, it was announced Bo Håkansson has entered into a partnership with Oticon Medical to take the BCI from a research project to a commercial product. The commercialisation of the BCI project is a long-term collaboration that will project with the aim to merge the excellent BCI clinical results with the experience and technologies from the Ponto bone anchored system.
References
1. Reinfeldt Sabine et al. 2015. Study of the Feasible Size of a Bone Conduction Implant Transducer in the Temporal Bone. Otol Neurot; January 2015.
2. Eeg-Olofsson M et al. The Bone Conduction Implant – first implantation, surgical and audiological aspects. Otol Neurotol; 2014:35(4):679-85.
3. Reinfeldt Sabine et al. The Bone Conduction Implant – clinical results of the first six patients. International Journal of Audiology, in press.
‘Spotlight on Innovation’ is an informative section to provide insight and discussion on recent advances in technology and research and does not imply endorsement by ENT and Audiology News.








