Audiology is a rapidly evolving field, with many exciting developments on the horizon. David Baguley identified some topics of interest, and asked some international experts ‘will it ever happen?’
Gene therapy for deafness
After years of development, gene therapy for sensory disorders is coming of age. Clinical trials using gene therapy to treat deafness and blindness are currently underway in the United States.
While the outcome of these trials is still a few years away, the trials have motivated numerous other translational studies that are attempting to restore function in animal models of human deafness. Because hearing loss can arise from many different causes, multiple approaches for hearing restoration will be needed. Current studies are focused on development of strategies to treat either acquired hearing loss or one of several forms of genetic hearing loss. A common issue is development of methods to deliver therapeutic gene sequences into the cells of the inner ear. One successful approach is to use nonpathogenic viruses to deliver gene sequences. This approach received approval from the USA Food and Drug Administration in spring, 2014 for a clinical trial to treat acquired hearing loss that results from death of the sensory hair cells. The strategy delivers a master control gene, known as Atoh1, which may function to convert supporting cells into sensory hair cells.
To treat recessive genetic hearing loss, scientists are focused on restoration of function by providing the correct gene sequence to nonfunctional inner ear cells. In this case, the correct gene may substitute for the native mutant gene sequences and may drive restoration of auditory function. To treat dominant genetic disorders, scientists must devise ways of suppressing expression of the mutant gene while allowing expression of the native correct gene sequence. Although gene therapy for hearing loss is still a number of years away from general clinical application, recent progress offers reason to be hopeful that these strategies may one day provide hearing restoration for tens of thousands of patients worldwide.
Further Reading
Géléoc GS, Holt JR. Sound strategies for hearing restoration. Science 2014; 9:344 (6184):1241062.
Kohrman DC, Raphael Y. Gene therapy for deafness. Gene Ther 2013;20(12):1119-23
Jeffrey R Holt
Fully implantable cochlear implant
The development of a fully implantable cochlear implant is a natural progression of current technology that would remove the stigma some patients experience from wearing the eternal processor and avoids problems associated with water exposure. This requires the functions performed by the current external components, namely the microphone and speech processor, to be incorporated into the internal package. In addition, a rechargeable internal power source is required. Early work in collaboration with Cochlear Ltd led to the development of the totally implantable cochlear implant [1] However, initial clinical experience found that speech perception was not as good as a standard cochlear implant.
In addition, internal microphones pick up noise from the patient, in particular swallowing and jaw movements. This is challenging to filter out. A potential solution to this problem was used in the Esteem Envoy, the first fully implantable middle ear implant. Mounting a sensor to measure the movement of the ossicular chain, which is usually intact and functioning normally in patients with cochlear implants, uses the natural microphone of the ear. A group from the University of Utah have developed a ‘microelectromechanical system accelerometer’ as a middle ear microphone that attaches to the umbo of the malleus, which shows promise in pre-clinical models [2].
The issue of power and speech processing is being addressed by a lab at Massachusetts Institute of Technology [3]. They have developed a new, low-power signal-processing chip that processes signals from a piezoelectric middle ear sensor to then stimulate a cochlear electrode. Their cochlear implant could potentially be recharged wirelessly in a few minutes using a charger that plugs into a smart phone. Other groups around the world are working on similar solutions and it is likely that a reliable totally implantable cochlear implant will be available in the near future.
References
1. Briggs RJ, Eder HC, Seligman PM, et al. Initial clinical experience with a totally implantable cochlear implant research device. Otol Neurotol ;29(2):114-9.
http://www.ncbi.nlm.nih.gov/
pubmed2008
2. Young DJ, Zurcher MA, Semaan M, et al. MEMS capacitive accelerometer-based middle ear microphone. IEEE Trans Biomed Eng 2012;59(12):3283-92.
3. Yip M, Rui Jin, Nakajima HH, et al. A fully-implantable cochlear implant SoC with piezoelectric middle-ear sensor and energy-efficient stimulation in 0.18μm HVCMOS. Solid-State Circuits Conference Digest of Technical Papers (ISSCC), 2014 IEEE International.
Mr James Tysome
A drug for tinnitus?
The most common cause of tinnitus is hearing loss. Most patients are offered hearing aids to help manage the symptoms, but these don’t cure the problem and they don’t work for everyone. The ear is a huge market that remains untapped by the pharmaceutical industry.
The good news is that a growing handful of small biotech companies across the world are now developing drugs for hearing-related disorders. Their efforts are diverse. Some are working on drugs that might prevent damage. Others want to treat damage that has already occurred. The diversity of approaches reflects the complexity of the auditory system. However, there are two broad directions: those that target the inner ear, and those that seek to modulate how the central nervous system processes the sound. Figure 1 illustrates this diversity of approach, as well as the different stages in the development pathway [1].
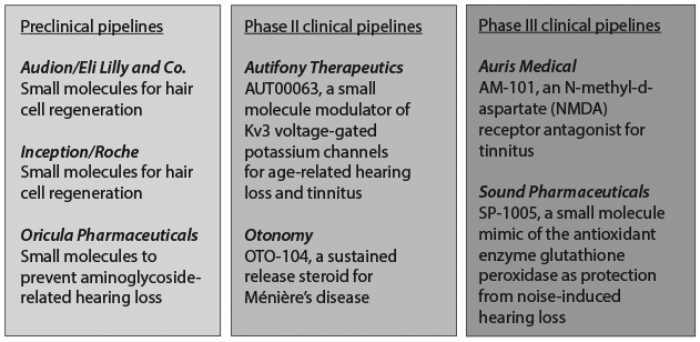
Figure 1: Clinical pipelines.
Drug discovery for hearing-related disorders is in its infancy and this poses a general challenge for the pharma industry. There are no precedents for successful drug trials in hearing, and so companies have to pioneer clinical trial design and conduct. A more specific challenge for tinnitus, is that in most cases the biotech’s therapeutic target is hearing loss. Although tinnitus is closely associated with hearing loss, this association is not a simple or straightforward one. Some people with troublesome tinnitus have audiometrically normal hearing and, conversely, many people with hearing loss do not report tinnitus. This means that the drug development process might miss opportunities to optimise the treatment of tinnitus.
The rise in interest and activity makes me confident that it’s more a question of ‘if’ than ‘when’ a drug for tinnitus will be available on the market.
References
1. Jarvis LM. Sound science. Chemical and Engineering News 2014;92(14):12-7.
Professor Deborah Hall
Automated audiometry?
Automated audiometry has the potential to address two current key pressure points on audiology services. In developing countries, which account for the majority of the estimated 360 million people globally with disabling hearing loss, there is a severe shortage of clinicians with audiological training. In developed countries, the shortage of audiologists is less serious, but most pronounced in rural and remote areas. However, here there are pressures on audiologists resulting from a growing elderly population, and changes in delivery models of rehabilitation services.
Automated audiometry has been around for more than 60 years but has re-emerged over the last 10 years on more advanced computerised systems with the potential to assist in addressing these two issues. A recent meta-analysis of all published studies has shown that automated audiometry is both reliable and accurate [1].
In developing countries, automated audiometers can be operated reliably by trained facilitators [2]. Results can be shared via secure communication channels to an audiologist at a central site for interpretation and advice. Video-conferencing can play a role in further hearing health counselling. However, delivery of hearing rehabilitation in the form of hearing aids remains a challenge, and is an important area for further development and innovation.
Automated audiometry may help to optimise clinical procedure in developed countries, allowing audiologists to focus on their primary skill sets of communication and rehabilitation specialists. Thresholds can be established automatically with the assistance of a trained facilitator, who may be undertaking other administrative or technical work.
Although two devices (KUDUwave, eMoydotnet, South Africa, and AMTAS, Audiology Incorporated, USA) have been the focus of validation studies in recent years, many audiometers are available with automatic features. However, only the two named devices have published data on their ability to determine both air and bone conduction thresholds.
Automated audiometry has to date not been thoroughly validated for children, and other solutions will be necessary for infants. However, recent developments in automated pure tone audiometry screening technologies [3] show promise that technological solutions may be found for screening purposes in young children also.
References
1. Mahomed F, Swanepoel DW, Eikelboom RH, Soer M. Validity of automated threshold audiometry: a systematic review and meta-analysis. Ear Hear 2013;34(6):745-52.
2. Swanepoel DW, Koekemoer D, Clark J. Intercontinental hearing assessment - a study in tele-audiology. J Telemed Telecare 2010;16(5):248-52.
3. Swanepoel DW, Myburgh H, Howe D, et al. Smartphone-based hearing screening with integrated quality control and data management. International Journal of Audiology 2014;1-9. (Epub ahead of print)
Professor Rob Eikelboom
Worldwide universal newborn hearing screening?
Apart from being able to cure or prevent hearing loss altogether, the possibility of worldwide early detection for infants with hearing loss through universal newborn hearing screening is at the top of the global audiology wish list. A world where every infant with congenital or early onset hearing loss is identified early and provided with appropriate and timely interventions to afford them the opportunity to develop optimally with benefits to be reaped over a lifetime for the individual and society.
At present the majority of the world’s infants have no prospect for hearing screening. Most are born in conditions where life-threatening communicable diseases overshadow disabilities like hearing loss. But this is changing. The number of global mortalities for children under five years of age has halved from 1990 to 2012 [1]. As global health indicators on quantity of lives saved continue to improve the importance of universal hearing screening to ensure optimal quality of life will become more pertinent.
Will it ever happen? I wonder what most would have answered to this question a few decades ago in a country like the United States of America where universal screening is now a reality. I do think I know what Marion Downs, the pioneer for universal infant hearing screening, would have answered to this question back when the Joint Committee on Infant Hearing was established in 1969 - it is not so much about whether it will happen but rather about when and how we make it happen. Back then there were no technologies that could facilitate mass screenings. Twenty years later, however, the first clinical screening technologies Otoacoustic Emissions (OAE) and Automated Auditory Brainstem Response (AABR) emerged which paved the way for universal screening. Who knows how future advances may enable us to overcome some of the current barriers that make this seem like an insurmountable task. Technologies are improving continuously and will rise to the occasion. What will be essential, are advocates for infant hearing loss that can ensure this priority is taken up on global healthcare agendas.
References
1. UNICEF (2014). Child Mortality.
http://data.unicef.org/
child-mortality/under-five
Last accessed September 2014.
De Wet Swanepoel
Vestibular perceptual threshold testing
Vestibular disorders are common and difficult to diagnose - about a third of patients with symptoms receive an uncertain diagnosis and / or a diagnosis that is unconfirmed by measurements or signs. While perceptual thresholds are widely used to diagnose hearing and visual disorders, quantitative clinical assays of motion-evoked perception are seldom, if ever, performed.
Recent findings [1-4] suggest that vestibular thresholds provide sensitive and specific quantitative diagnostic measures. As just one specific example, patients diagnosed with vestibular migraine show roll tilt thresholds that can be about four times lower than for normal subjects or migraineurs, which could help distinguish vestibular migraine from Meniere’s disease.
Other reasons for optimism regarding perceptual threshold diagnostic testing are:
- It can be used to assess all peripheral end-organs with one methodology. This contrasts with (and may complement) the current clinical approach that combines a wide variety of measures.
- Thresholds provide a straightforward way to test central vestibular processing, alongside peripheral function.
- Threshold testing uses motion stimuli (i.e. rotation and / or translation) that provide more direct functional assays than tests that rely on thermal, noise, or vibratory stimuli.
- Thresholds are determined using small stimuli. Since the brain receives little information for near-threshold motion, it is unlikely that the brain’s compensatory mechanisms affect thresholds. Furthermore, small motions minimise motion sickness.
- Threshold methods mimic standard audiology methods. Hence, physicians and audiologists already have the background and experience to perform and interpret this test.
One potential concern is that perceptual threshold testing would require a new testing facility. Mitigating this concern is that only threshold-scale stimuli are required so the test could be set up in a small office. We also emphasise that all aspects of central and peripheral vestibular function could be tested using a single device. If marketed, such a device will likely cost less than the total cost of the array of devices used today.
References
1. Lewis RF, Priesol AJ, Nicoucar K, Lim K, Merfeld DM. Abnormal motion perception in vestibular migraine. Laryngoscope 2011;121(5):1124-5.
2. Valko Y, Priesol AJ, Lewis R, Merfeld D. Vestibular labyrinth contributions to human whole-body motion discrimination. Journal of Neuroscience 2012;32(39):13537-42.
3. Agrawal Y, Bremova T, Kremmyda O, et al. Clinical testing of otolith function: perceptual thresholds and myogenic potentials. J Assoc Res Otolaryngol 2013;14(6):905-15.
4. Priesol AJ, Valko Y, Merfeld DM, Lewis RF. Motion Perception in Patients with Idiopathic Bilateral Vestibular Hypofunction. Otolaryngol Head Neck Surg 2014;150(6):1040-2.
Daniel M Merfeld and Richard F Lewis











