The British Cochlear Implant Group’s candidacy working group recently ran a national exercise, working towards a consensus on candidacy for cochlear implantation in the UK. Padraig Kitterick and Debi Vickers were instrumental in this exercise, and in the article below, they explain the process involved in achieving this consensus.
Cochlear implants restore important aspects of hearing in people whose degree of hearing loss means that they struggle to communicate using spoken language, even when using conventional acoustic hearing aids. The provision of cochlear implants in the UK currently follows guidance from the National Institute for Health and Care Excellence (NICE) [1].
NICE recommends cochlear implants for adults and children with profound deafness in both ears. To be eligible, individuals must also receive insufficient benefit from acoustic hearing aids, a criterion that is determined using the Bamford-Kowal-Bench (BKB) sentence test administered under quiet listening conditions.
Recent studies have concluded that UK candidacy criteria are some of the most restrictive in the world [2] and appear to be at odds with the views of patients and clinicians [3]. If candidacy criteria are to be revised, there will need to be supporting evidence to confirm that implantation is likely to be effective in other patient groups. Evidence-based approaches to developing new criteria for candidacy have been developed, and applied notably in severe-to-profoundly-deaf children [4].
However, there is insufficient evidence to develop criteria using such approaches in many other patient groups. In the absence of such evidence, consensus methods provide a way to summarise the views of patients and clinicians, identify where they agree on matters of candidacy, and to set the priorities for future research.
The British Cochlear Implant Group (BCIG) represents providers of cochlear implantation services for adults and children across the UK. The BCIG candidacy working group recently ran a national exercise to find out if there was agreement among the major stakeholders in cochlear implantation on who should be able to receive a cochlear implant on the NHS in the UK.
The exercise involved over 30 organisations including cochlear implant centres from around the UK, organisations that represent those with hearing loss and those who use cochlear implants, organisations that represent the clinical professionals involved in providing cochlear implantation, organisations conducting research on cochlear implantation, manufacturers of cochlear implants, and those who commission cochlear implantation services.
A stakeholder panel made up of representatives from those organisations was asked to consider whether cochlear implantation is appropriate for a range of different patient scenarios (Figure 1). Appropriate in this context meant whether the benefits of cochlear implantation were thought to outweigh the risk of any harms. The process followed an established methodology for studying the under or over use of medical and surgical procedures [5].

Figure 1. Photograph of the face-to-face meeting at which the range of patient scenarios were discussed and representatives from stakeholders voted on statements about candidacy.
The choice of patient scenarios for consideration were based on responses to a survey made available online via a dedicated website:
http://www.cicandidacy.co.uk.
The survey asked for views on who should be able to receive a cochlear implant. The survey was completed by 161 individuals including 61 cochlear implant users and parents of implanted children, 38 people who had been refused an implant or would like one, and 62 clinicians and researchers.
Over 20,000 words of comments from these individuals were analysed to see what factors were considered important when deciding who should be able to access cochlear implantation. These factors included:
- If they have hearing loss in one or both ears
- What level of hearing loss they have
- If their hearing loss is likely to get worse in the near future
- If they have already received one cochlear implant
- How much difficulty they have understanding speech in quiet and in noisy situations
- If they are children or adults
- If they acquired their hearing loss early or later in life
- If they have tinnitus (ringing in the ears) or not
- If they have any other significant sensory problems, e.g. limited vision.
The combination of these factors created scenarios for over 600 different patient groups. Each member of the stakeholder panel first considered their views about the appropriateness of cochlear implantation in each of these scenarios independently, and then met in person to discuss them. The stakeholders were allowed to change their views after these discussions. The panel provided over 22,000 ratings of the patient groups over the course of the exercise. The results were used to put each patient group into one of three categories:
- ‘appropriate’, which meant that the panel thought that the benefits of cochlear implantation to the patient outweighed the risk of any harms
- ‘uncertain’, which meant that the panel could not agree if cochlear implantation was appropriate or not
- ‘inappropriate’, which meant that the panel thought that the risk of harms from cochlear implantation outweighed the benefits that the patient might receive
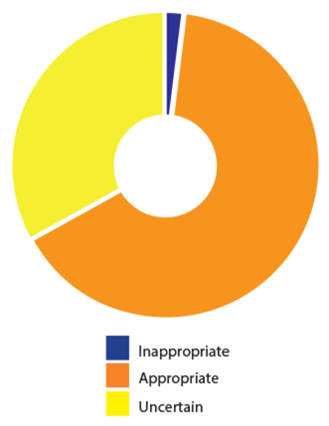
Figure 2. Summary of the overall results from the evaluation of the appropriateness of cochlear implantation in 600 different patient scenarios.
The panel agreed that cochlear implantation was appropriate in almost 400 of the 600 patient groups that were considered (Figure 2). The panel viewed cochlear implantation as inappropriate for only two patient groups but there was uncertainty over the remaining groups. Where there was uncertainty, the data showed that it was not because of a mix of appropriate and inappropriate ratings but rather that people were unsure about the effects of cochlear implantation in those patient groups. Appropriateness was seen to depend on many factors and the results therefore suggested that patients in whom implantation is appropriate could not be defined adequately using a small number of well-defined criteria or cut-offs, as is currently the case in NICE guidance [5]. However, the results confirm that the range of patient groups in which cochlear implantation is considered appropriate is considerably broader than the range of groups who are currently eligible according to NICE guidance.
The principle conclusions from the exercise are that:
- Cochlear implantation is considered appropriate for less profound degrees of hearing loss than currently permitted according to NICE guidance.
- Cochlear implantation can be appropriate where the degree of hearing loss is different in the two ears, and in patient groups where only one ear would be considered appropriate for implantation.
- Cochlear implantation is not only considered to be appropriate when a patient receives insufficient benefit from their hearing aids when listening in quiet, but can also be appropriate when hearing aids provide insufficient benefit only when listening in background noise or where speech understanding is not possible or appropriate to measure.
- Cochlear implantation is not only considered to be appropriate where the primary motivation for treatment is the restoration of speech understanding but can also be appropriate where it is for the alleviation of tinnitus.
Finally, while current guidance only recommends unilateral implantation in adults, the stakeholders agreed that bilateral cochlear implantation can be appropriate in adults but its appropriateness varies based on factors including age, degree of hearing loss, whether deafness was acquired early or later in life, whether the patient has an additional sensory impairment, and whether there is a known risk of hearing declining further.
It is important to acknowledge that consensus around the patient groups in which cochlear implantation is considered to be appropriate is not a replacement for high-quality evidence for its clinical effectiveness and cost-effectiveness. The fact that any recommendations made by NICE have direct implications for commissioning means that they will always seek to be guided by clear evidence that cochlear implantation leads to patient benefit and is an efficient use of limited NHS resources. However, an exercise such as this is still informative because it clearly identifies those patient groups for which there is already strong agreement about candidacy across a broad range of stakeholders.
The results also identify gaps in current evidence that future research should prioritise in order to provide NICE with the information it requires to form recommendations on patient groups that are currently not eligible for cochlear implantation on the NHS in the UK.
References
1. National Institute for Health and Care Excellence (NICE). Cochlear implants for children and adults with severe to profound deafness. 2009;
https://www.nice.org.uk/guidance/ta166
2. Vickers D, De Raeve L, Graham J. International survey of cochlear implant candidacy. Cochlear implants International 2016;17(S1):36-41.
3. Vickers D, Kitterick P, Verschuur C, et al. Issues in Cochlear Implant Candidacy. Cochlear Implants International 2016;17(S1):1-2.
4. Lovett RES, Vickers DA, Summerfield AQ. Bilateral cochlear implantation for hearing-impaired children: criterion of candidacy derived from an observational study. Ear and Hearing 36(1):14-23. 5. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. (2001). The RAND/UCLA appropriateness method user’s manual. 2001; Santa Monica, CA, USA; RAND Corp.
Acknowledgements
We wish to acknowledge the input of the other members of the BCIG candidacy working group: Carl Verschuur (Southampton Auditory Implant Service), Caroline Leal and Louise Jenkinson (Guys and St. Thomas’ Hearing Implant Centre), and Fiona Vickers (Cochlear Implant Department, The Royal National Throat Nose and Ear Hospital). We wish to thank all the stakeholders for their contributions. The costs of the exercise were met through support from the NIHR Nottingham Biomedical Research Centre, UCL Speech Hearing and Phonetic Sciences Department, and the BCIG.





