Background
endoscope-i (e-i) Ltd was incorporated into Companies House on December 3rd, 2012 following eight months of developing our first project, a simple iPhone adapter for endoscopes. Of the three founding shareholders, two are ENT surgeons and one a lecturer in engineering from Aston University, UK.
Starting out without any business or marketing experience was certainly challenging, but a deep understanding of our respected areas of expertise has been our most valuable asset. In eight months of trading the company has turned over just £5000, but with capital investment more than triple this amount we are a long way from being profitable.
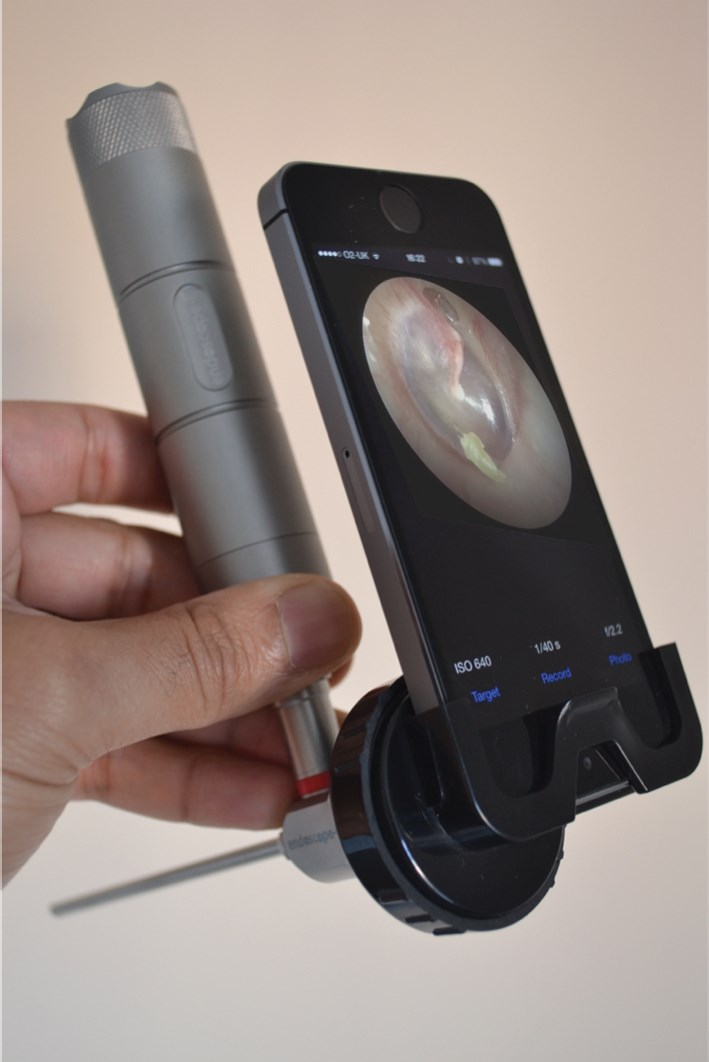
Figure 1a: The world’s first endoscopic app / adapter iPhone system, ‘endoscope-i’.

Figure 1b: Photo documentation of a patient’s larynx in clinic.
The company was founded on the philosophy of ‘engineering simple solutions to previously complex problems’, highlighting our ambition to create purposeful innovations. It did not take long to realise the unique selling point (USP) of our product would be to create the world’s first endoscopic camera app for the iPhone, offering a complete solution for endoscopic mobile imaging (Figure 1a,b), although our initial desire was simply to design a cheap and easy system of taking great endoscopic images and videos using technology that most of us own already (a smartphone), the e-i system has subsequently been used in ways we never predicted (Figure 2a,b).
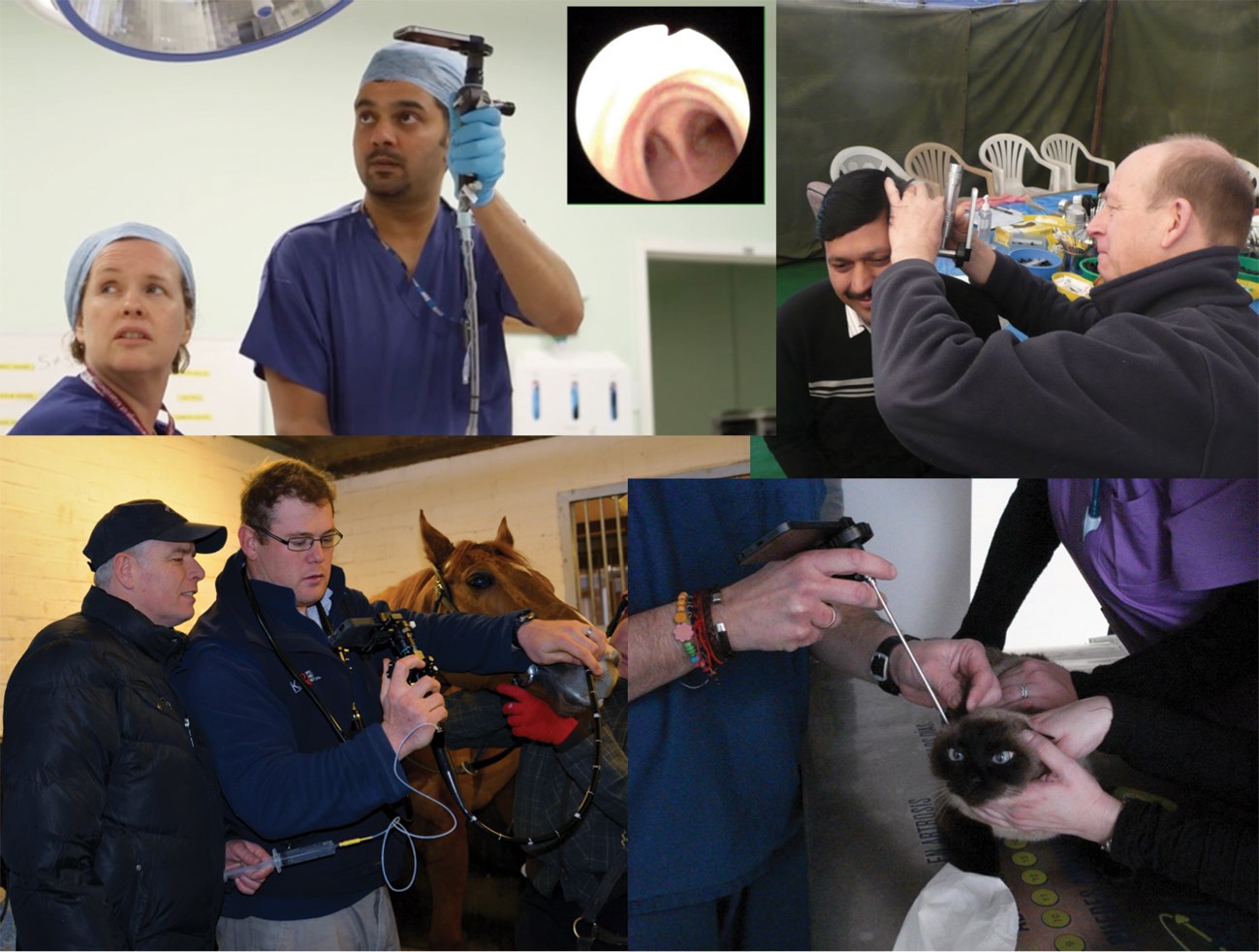
Figure 2a: From top left clockwise: Dr Ajit Bhat performs iPhone fibreoptic intubation as a colleague watches the streamed live video on the HD monitor (the carina on inset), Mr Mike Smith at the Nepal ear camp, Dr Francisco Martinez Gomariz performing feline iPhone otoscopy and Dr Lewis Smith performing equine iPhone laryngoscopy.

Figure 2b: Ms Seema Yalamanchili uses the e-i at Everest base camp for the ‘Xtreme Everest2’ team.
This article describes our journey through the barriers to innovation in healthcare. Through the quotes of experts, our team describes some pearls and pitfalls of creating a medical innovation.
Think of an idea
“There is no shortage of remarkable ideas, what’s missing is the will to execute them.” - Seth Godin
A great idea is just the start. Importantly, it has to be more than just one person who thinks the idea is great. The difficulty with a new invention is that the advice of many is to “keep the idea to yourself” to protect it and prevent it from being ‘stolen’ or ‘copied’. How then, is it possible to know whether your idea is indeed a good one? A non-disclosure agreement (NDA) is the official method of sharing early ideas whilst protecting your interests and we used these extensively when discussing our ideas with third parties. It’s no surprise with this early obstacle that the majority of great ideas remain as just that.
Fortunately the world of medicine is abundant with experience and expertise and no doubt through one’s career you will have got to know a few particular trustworthy colleagues. These are the individuals in which to confide. You will receive an honest opinion; something you will not receive from many of the ‘build your invention’ companies online, motivated by your money rather than your success.
Create a philosophy, and then stick to it
“You must be the change you wish to see in the world.” - Mahatma Gandhi
It is all too easy to get carried away with a great idea but it is important to remain focused. By creating a company philosophy we were able to stick to the fundamental elements that inspired the concept of endoscopic mobile imaging. When modifying or expanding our ideas we refer back to our philosophy of ‘engineering simple solutions to previously complex problems’. If any of these conditions are not met, we simply stop and start over again. A considerable amount of time was dedicated to creating our philosophy. Our vision is based around how we wish to see the world innovate in the health service, with a focus upon simplicity. In the words of Einstein, “Everything should be made as simple as possible, but not simpler”.
Build a team
“Surround yourself with people smarter than you” - Russell Simmons
“You don’t build a business, you build people and then people build the business.” - Zig Ziglar
By exploring new ideas you will have already assembled a small team, with each member harbouring expert knowledge that is important to utilise. Our company secretary, Mark Prince, used his academic engineering experience to give us an insight into the world of biomedical material science, computer aided design (CAD), stereolithography (SLA) and injection moulding as well as offering a different perspective on endoscopic mobile imaging from a non-medical scientific point of view. For the purpose of this article Mark provided us with an insight into working alongside clinicians to develop innovative medical solutions:
“Setting aside the obvious value of the experience of developing a novel product through to market and establishing a commercial base, there are distinct advantages in developing and engineering new products when your colleagues are also the end-users. Working closely together with practising clinicians when developing medical products provides rich resources in terms of user perspectives, application-specific expertise and knowledge of operating healthcare environments. Direct access to enthusiastic, visionary but pragmatic partners facilitates instant feedback and rapid iteration keeping the design process swift and focused.
Since working with Ajith and Chris I have been afforded great insight into the issues, pressures and priorities of those working in clinical practice. Drawing upon their experience and the strength of their collaborative networks I now enjoy access to real problems and concerns from the healthcare sector upon which I can align my research efforts in order to provide effective and high-impact solutions that are ultimately practicable.”
Similarly when developing the user interface (UI) for our endoscope-i, e-i Pro and iBeam Apps, our software developer, Simon Pargeter, introduced us to ‘Xcode’ and the challenges of writing code under the regulations of Apple. In return Simon gained an invaluable insight into the challenges of medical IT to further understand some of the reasons that led to the demise of the NHS National Programme for IT. Simon shares his viewpoint below on developing the e-i system:
“All too often projects are driven by a layer of people who don’t use the product directly, leading to feature creep and conflicting ideas. Working with Ajith, Chris and Mark as a small agile team providing fast responses to ideas and continuous testing of the app truly demonstrates that better products can be produced when everyone is part of the implementation.
Knowing that any app release issued is going to be tested immediately by an end user and the feedback and insights are truthful and relevant to the functionality not only accelerated the product development, but also allowed me to immerse myself in their field of expertise (if only at the shallow end). Understanding the needs and pressures of the end user is what differentiates a great app from the rest.
Medical device apps are still in their infancy, but thanks to being part of the endoscope-i team, an exciting future to be part of.”
Create a prototype
A landmark occasion is seeing your idea come to life as a prototype. The use of CAD will ensure that sizing specifications are correct and do provide a reasonable insight into the finished look and feel of the product. For product development the process of 3D printing has had a significant impact in rapidly converting computer images into a plastic prototype within a few hours. We used a two-part construction to create the first endoscope-i adapter using Solid Works 2012 [1]. The app was built using Xcode for Mac and distributed to each member of the team using an application well known to app developers called ‘Test Flight’. This facilitated beta testing of apps before they were ready for App Store submission. Any ‘bugs’ found in the software were reported back to the software developer.
Know the rules, push the boundaries, test the idea and ask the experts
“Successful people are the ones who are breaking the rules.” - Seth Godin
We don’t encourage deliberately going against regulation but it is important to read and learn the small print, whether this is the local hospital protocol for imaging with mobile devices, national General Medical Council (GMC) good medical practice guidance on obtaining medical images of patients [2], or indeed international publications from the governing authorities. Guidelines are, in some degree, open to interpretation. We presented the e-i project in its infancy at the Royal Society of Medicine in April 2013 at an ‘Apps in Healthcare’ forum. These meetings provide the perfect opportunity to discuss with the key stakeholders in NHS healthcare regulation, such as those responsible for the NHS app store and the head of new and emerging technologies at the Medicines and Healthcare products Regulatory Agency (MHRA), to unravel the meaning of the regulations.
One such group we forged a relationship with was HANDI health. HANDI is a not-for-profit company created to enable learning and partnerships in health and care and to work to create a community in which the promise of apps can be fully realised [3]. HANDI is for patients, carers and the public, health and care professionals (practitioners and managers), app developers, academia, industry, government and the investor community and anyone else who supports the ‘app paradigm’.
Apps in healthcare have grown exponentially in 2013 to the extent that regulatory bodies such as the Food and Drugs Administration (FDA) and MHRA were forced to issue guidance. The FDA did so in September 2013, outlining the definition of a mobile app as a medical device. The key determinants are: the “intended use” of the app and potential risks to patient safety if the mobile application is used incorrectly [4]. The MHRA however, are yet to officially issue guidance on medical apps. Their current regulations on medical devices, the Medical Device Directive 93/42/EEC [5] is organised into three categories: class I, II or III. Currently medical apps do not fit into any of these and it may be, in the future, we see a class ‘0’ category for medical apps. For now, class I regulation is probably the best suited and again focuses on “intended use” of the application; “Software that does not have a medical purpose, but is placed on the market for a more general purpose will not be a medical device, even if a user decides to use it for a medical purpose”.
Our advice would be not to let these regulations hold back creating a prototype. Publicise your work in as many different ways as possible through social media, journals, conferences and magazines. To date e-i has featured in Clinical Otolaryngology, E-Health Insider (for which we were finalists in the ‘IT Healthcare Product Innovation’ category), and MedGadget. In doing so we attracted the attention of a number of companies interested in working with us to explore the concept of endoscopic mobile imaging, of note Apple, who invited us to showcase the product at their Regent Street Store in London and Single Use Surgical (SUSL), who currently manufacture and market the e-i adapter.
When it came to manufacturing we were in a favourable position of having a team member experienced in engineering who could communicate in common terms with the team at SUSL. This was particularly beneficial when it came to re-designing the e-i adapter for mass production as an injection moulded product, as Matthew Tulley (Director of SUSL) explains:
“When we saw the endoscope-i prototype, we thought it was a great idea. We were able to use our experience of design, tool making, manufacturing and clinical sales to help the product get to market in a timely and cost effective manner. The product obviously wouldn’t have been born without the endoscope-i surgeons and designer, or the app development skills of Simon. The clinical input from endoscope-i has been key in making sure the all- important user interface, training and user feedback is incorporated in further developments. You can’t ask for better credibility when selling to a surgeon than ‘designed by surgeons’.”
The final adapter design was significantly different to the original SLA version reiterating the valuable advice given to us by a patent advisor to “only consider patency when the design is finalised”. By this stage we had already published much of our developments making patenting impossible. However we found the notion of being ‘first to market’ carries a lot of kudos in the world of innovation.
Often, the first question anyone asks us is “have you patented the product?” but we increasingly ask ourselves, where is the need to do so? The amount of time, effort, research, meetings, development, publications and knowledge etc. is difficult to replicate and why would it be commercially viable to copy when the final product is available at such a low cost?
Take the plunge and invest some money
“A business has to be involving, it has to be fun and it has to exercise your creative instincts.” - Richard Branson
For most entrepreneurs this is the critical point when a significant investment of money is required. For us, it was also the moment we decided to incorporate e-i Ltd as a business into Companies House in order to protect our own interest. Intellectual property (IP) exists in many different forms but you cannot protect an idea. By this stage we had amassed a number of adapter prototype designs, a company logo and a name. Purchasing our website domain was one way of securing naming rights. Product and logo designs can be protected for a small fee on the ipo.gov.uk website. Having yet to turn over any money, it is a difficult decision to balance out the money to spend on protection. What’s the point in paying to protect a bad idea?
We took the ‘plunge’ because we enjoyed the project immensely, and it allowed us to explore a creative avenue in developing our website, marketing and promotional materials and packaging. By investing our own finances we were particularly stringent in resource allocation. All too often medical innovations seek external funding. Aside from the obvious challenges of IP sharing one may not be as careful with money they have no ownership for.
Look to the future, learn from the past
“If you do something and it turns out pretty good, then you should go do something else wonderful, not dwell on it for too long. Just figure out what’s next.” - Steve Jobs
“Your most unhappy customers are your greatest source of learning.” - Bill Gates
We have no idea of the commercial ‘shelf life’ of our products, which is why it is imperative that we learn as many lessons as possible to take on to the next project. For us, e-i was a platform to innovate in healthcare and the accumulation of knowledge in taking a product from idea to market has far outweighed any financial gain. In the last few months we have been assisting in the development of lens optics specifically for use for the e-i system alongside the expertise of Microscopix Ltd and will release the world’s first app to mirror live camera images between iOS devices, iBeam.
Our experience has taught us that the key to successfully marketing an innovation is to get the early adopters to believe in you and the product, as:
“People don’t believe what you tell them. They rarely believe what you show them. They often believe what their friends tell them. They always believe what they tell themselves.” - Seth Godin
Further information
For further information about endoscope-i, visit www.endoscope-i.com.
References
1. George A, Prince M, Coulson C. The ‘endoscope-i’: a mobile solution for endoscopy in otolaryngology. Clin Otolaryngol 2012;38:104-6.
2. Making and using visual and audio recordings of patients. General Medical Council- Supplementary guidance; 2011. Available at:
http://www.gmc-uk.org/Making_and_using
_visual_and_audio_recordings_of
_patients.pdf_58838365.pdf
Accessed March 2nd, 2012.
3. HANDI Health. About us. 2013. Available at:
http://handihealth.org/about-2/
Accessed April 20th, 2013.
4. Mobile Medical Applications. Guidance for industry and Food and Drug Administration Staff. 2013. Available at:
http://www.fda.gov/downloads/MedicalDevices/
DeviceRegulationandGuidance/Guidance
Documents/UCM263366.pdf
Accessed September 25th, 2013.
5. Ebenezer N. The regulation of software as a medical device. 2013. Available at:
http://www.mchiec.org.uk/assets/files/
Healthcare_Apps_-_Maximising_Impact_2013_-NeilEbenezer.pdf
Accessed April 20th, 2013.
Declaration of Competing Interests: AG and CC were involved in the development of endoscope-i.






