Why the iAudiometer™?
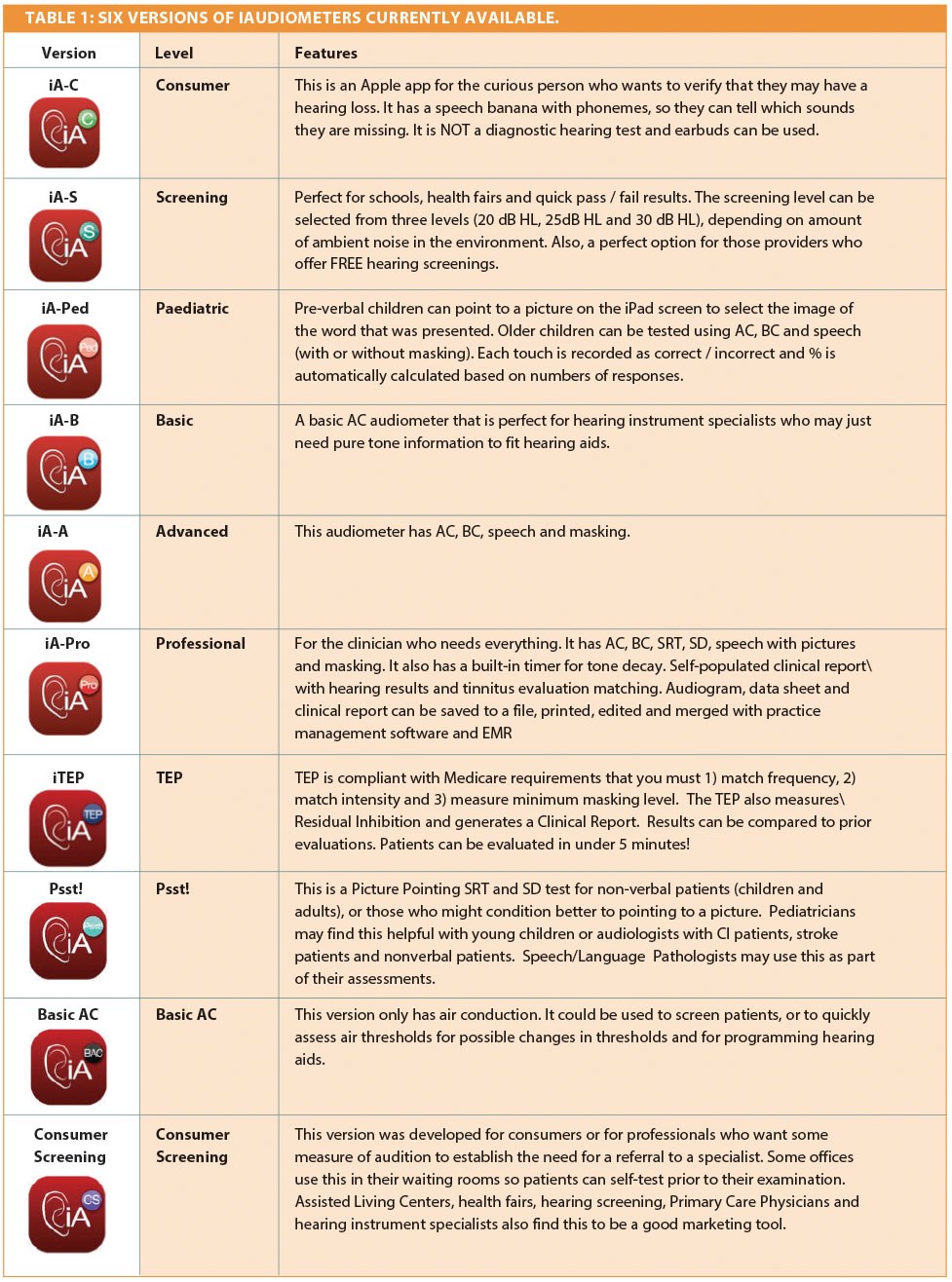
We have developed six versions of a new software called iAudiometer™ that performs an array of different audiometric tests using an iPad with standard transducers (TDH-39 headphones, B-71 BC, aural domes, or inserts) (Figure 1; Table 1). Our team consisted of one audiologist and two biomedical engineers. We asked this question: “How can we make a useful clinical tool that would be 1) more cost effective, 2) easily deployed and most importantly 3) clinically useful?”
We came up with the idea of building an audiometer in software and making it available on an iPad and iPhone. By utilising Apple’s hardware, the device would have the added benefits of lower costs and added mobility, while still maintaining all the functionality of a traditional audiometer. Our motivation came from the need for a cost effective, light weight, small device that would perform any function that a commercial audiometer could perform and meet ANSI S3.6-2010 standards.
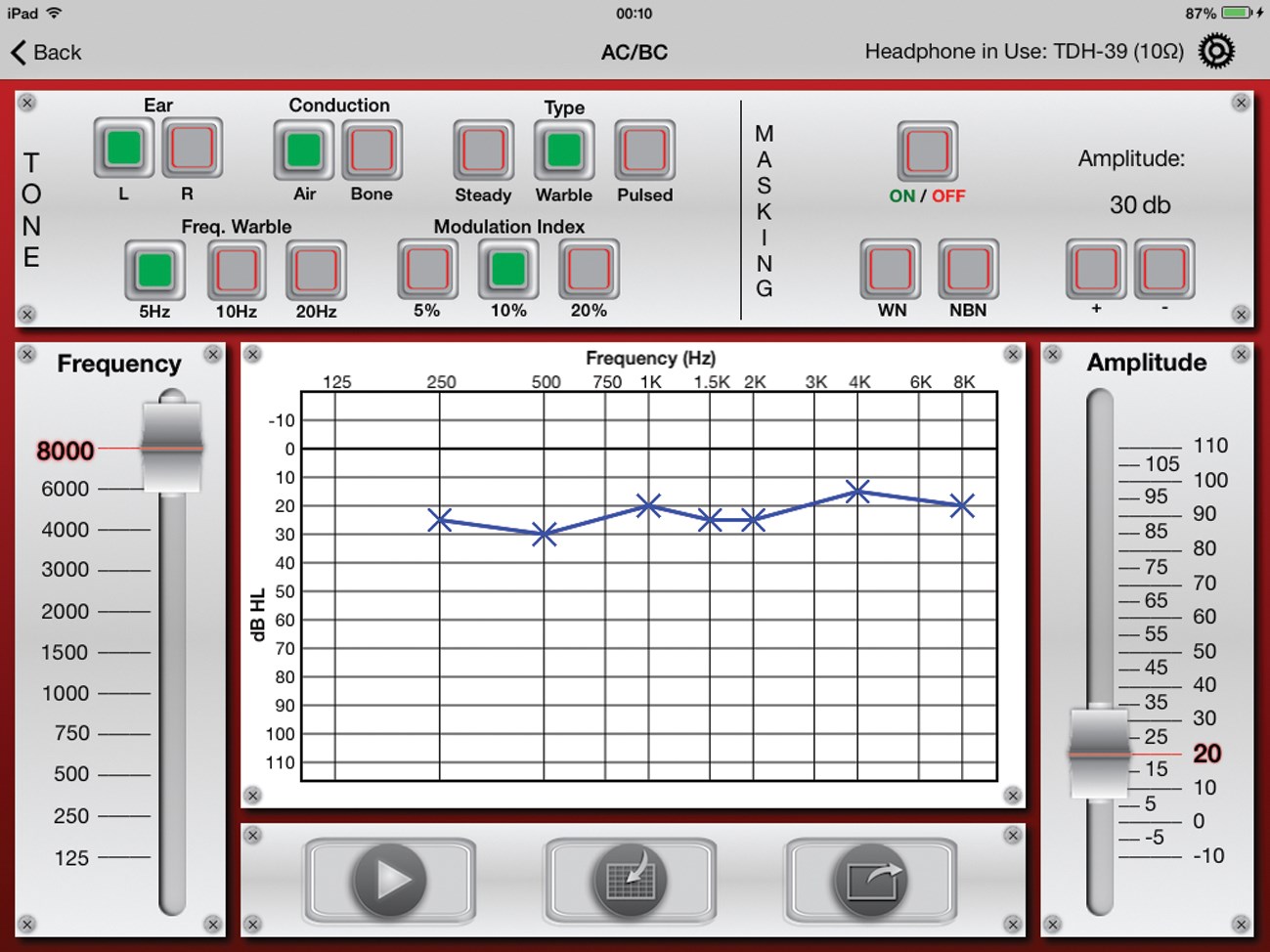
Figure 1: iAudiometer™ main screen.
With reimbursements going down and expenses going up, hearing healthcare providers in the USA are finding their profit margins shrinking. We don’t always need every hearing exam to be done in a 10’x12’ room with a sound booth. That’s expensive real estate, plus the equipment! You need that environment for specific cases, but not for every patient.
iAudiometer™ can be used in, or for
- The operating room
- Any available clinic room
- Hospital in-patients
- Satellite offices
- Nursing homes
- Hearing screenings in public schools
- Health fairs
- Pre / post-employment evaluations
- Monitoring ototoxic drugs
- Generating more referrals from paediatricians and primary care physicians.
The iAudiometer™ can also be used in your sound booth room as well!
Addressing intellectual property, regulatory affairs and reimbursement issues
To meet intellectual property requirements, we filed a trademark for our product, filed a provisional patent and purchased the domain name for the product.
To comply with regulatory affairs we contacted the US Food and Drug Administration for current regulation status of mobile applications for class II audiometer medical devices (otolaryngology). The decision was that audiometer mobile applications are exempt from a 510(k) if they are by prescription only.
In terms of reimbursement, our iAudiometer performs all of the functions for audiometric hearing testing using an audiometer according to ANSI S3.6-2010 standards. Each audiology current procedural terminology (CPT) code that uses an audiometer to establish hearing function using frequency, intensity, masking and speech can be billed for each specific code.
Determining commercial viability
We conducted an online survey to 2,704 hearing healthcare professionals using ConstantContact (www.constantcontact.com) (at time of submission we were still gathering responses). From experience we knew that with proper transducers:
- Mobile audiometers can serve multiple offices more efficiently
- Mobile audiometers are more cost effective
- Mobile audiometers can be used in public school hearing screening programmes more efficiently and at a lower cost
- Mobile audiometers can be used in any unoccupied room.
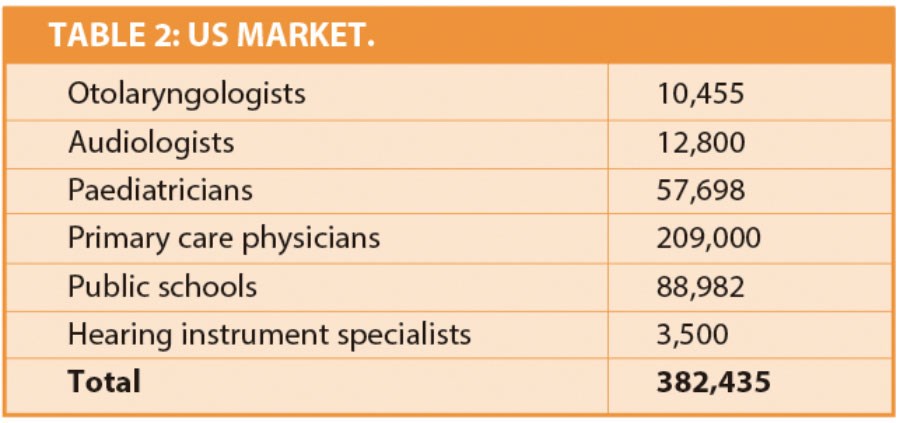
We established a count of the various entities that currently use audiometers and may consider using our product (Table 2).
Product verification and validation
ANSI S3.6-2010 is the standard for audiometers. Our product performance was verified and validated using an Apple iPad with our software and a B&K audiometer calibration system.
Our biggest obstacle: lack of funding
Funding has been our biggest problem. We have been able to use personal funds and sweat equity to cover initial costs. Investors are hesitant to invest in companies that have not yet produced revenue. Venture capital typically want to see revenue over a two to three year period of time. Strategic partners may be a source at some point in time.
Our biggest mistake: diversified team
Looking back, we would agree that trying to develop complex products without having enough team members prevented us from progressing as quickly as we should have. Once the opportunity presented itself of joining forces with the missing components, we then ramped up quickly. Within a few months of bringing the team together, we have developed six products and have three more in development.
Advice we can offer to others Frugal marketing
The first question we asked is, how were we going to market and sell our products? The key is frugal marketing, and here is our advice on how to reach your target market(s).
Use what you have. We began by compiling a list of world-wide distributors who sold audiometers, accessories (transducers) and offered calibration services. These distributors could not only sell our software, but they could upsell their transducers and also perform annual calibrations (win-win). We also used the Hearing Review’s World Directory to locate our potential distributors. It was important to have their email addresses (free marketing), so when there was not an email address we obtained it from their website. The advantage of using distributors is that they have a customer base and a sales force in place to introduce your product and make additional sales.
Networking. Start with your current contacts and use them to help you grow your list. Set up a Facebook account and build your list of ’friends’. We also used LinkedIn. It’s a great way to network with people who understand your specialty. We joined numerous ‘groups’, which were related to hearing healthcare so we could ‘follow’ them and join in their ‘discussions’. In our particular case we covered biomedical engineering, otolaryngology, audiology, paediatrics, primary care and other related groups. We invited them to ’connect’ with us and hopefully ’endorse’ us.
Inform your customers. We used the email addresses we had gathered from our networking groups. We used ConstantContact, because they offer a variety of tools that will get you started (email templates, surveys, polls, contact databases) and they will also make sure that you are using ’opt-in’ email addresses. We also conducted a survey to try and determine factors such as level of interest, need, price points, product features, etc.
The Internet has certainly changed the way we live our lives. We are a global economy and we can now access markets around the world. In doing our research on distributors we were surprised at the extent of their global reach. As a result, we are planning to market our products in many languages.
YouTube is another way to economically distribute information on your products. Instructions on use of the product, technical information, FAQs and any other specific topics your customer may find helpful. It is easy to make your own videos and there are many apps available to help you edit them. You also have the ability to add audio, pictures and captions. We will ask our distributors for their help in providing captions for our videos in their local language.
Summary
In this article we have covered some of the steps that most entrepreneurs would take, that are common to many start-ups. However, each situation has its own specific characteristics. Here are some ’take away’ questions that we didn’t include in the above discussion, but that you, as an entrepreneur, should ask yourself:
- Do you have the expertise?
- Have you consulted others?
- Have you developed a detailed plan?
- Are you a risk taker?
- Will you and your family be willing to make personal sacrifices?
- Do you have enough funding to get started?
- Are you willing to work long hours?
- Do you have a back-up plan?
We wish you great success in your next adventure!
Declaration of Competing Interests: DH, AC and GL have shares in Melmedtronics, who funded the development of the iAudiometerTM.






