Background – surgical technology and otolaryngology
An estimated 234 million major surgical procedures are performed annually worldwide. This requires the interaction of multidisciplinary teams with varying contributions of surgical technology and therefore makes surgical procedures prone to multiple sources of error. In that context, it is perhaps not surprising that surgical errors comprise nearly two thirds of in-hospital adverse events [1, 2].
Advances in surgical technology have played an important role in improving patient care by expanding surgical options and improving patient safety in the operating environment. This has meant a reduction in post-operative morbidity and mortality. However, the rising use of surgical technology also increases both procedural complexity and risk of equipment failures.
As one of the larger and technologically evolving surgical subspecialties in the NHS, otolaryngology is an area to which studies of error and technology may be particularly relevant. New techniques and technologies are being made available to clinicians on a regular basis: these include developments in sinus surgery including functional endoscopic sinus surgery (FESS) and balloon sinoplasty, lasers and more recently the use of robots.
Surgical technology and error – what we can learn?
Collecting data on medical errors in general is challenging. This is reflected in the heterogeneity of results reported in the literature [3] and one has to take into account the risk of bias from variability in study design (retrospective versus prospective studies), error classification, etc. However, bearing these in mind and by adopting clear criteria and definitions, we have found that useful insights for clinical practice can be gained from systematically studying intraoperative errors directly and amalgamating experience through literature review [3-5]. The body of evidence for error relating to technology is sparse in comparison to error studies in general and represents an important area of research.
In a recent systematic review we looked at the role of equipment failures in the operating room (OR); we reported in 28 eligible studies documenting error in the OR from a total 18,848 surgical procedures across a range of surgical specialities [3]. It was striking that, although total error varies very widely, errors due to equipment and technological failures consistently formed a substantial proportion of total reported error – median 19.3% (IQR 14.3%–29.8%) of total errors and this corresponded to a median per-procedure equipment-failure rate of 0.9 (IQR 0.3-3.6) errors / procedure.
We were able to define three main types of surgical technology error, which account for equipment failures in roughly equal proportions:
- Availability: where the correct equipment required for performing part or all of the operation is not available when needed
- Configuration and settings: problems with the correct set-up of the equipment preventing normal functioning
- Direct malfunctioning / failure.
This demonstrates the varied aetiology of equipment-related errors, but also highlights the potential for mitigating a large proportion of these errors at various stages before the operation.
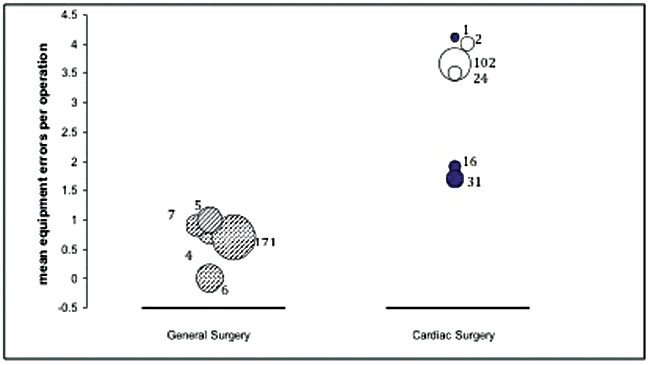
Figure 1: A comparison of error-rates in five general surgical and six cardiac surgical studies.
Each bubble represents a single study and its area represents the number of operations considered in that study.
Dark bubble = adult cardiac surgery, white bubble = paediatric cardiac surgery.
There are significant differences in error-rates between specialties: for example we found that, looking at studies of similar design (prospective, direct observer method) there is a striking difference in total and equipment-related error in general versus cardiac surgery (Figure 1). In our own studies of over 400 hours of vascular procedures, we found the highest rates of failures consistently occur in procedures that rely on advanced technology and multidisciplinary teams [4]. Other technology-reliant specialities, such as ENT, which are increasingly reliant on specialist equipment and high technology, may also be more susceptible to equipment-related error.
The most robust method of comprehensively and systematically capturing intraoperative error rates seems to be through prospective studies using direct observation. Assessment of surgical equipment errors may also be made in proxy via examining reports of equipment failures leading to patient harm and subsequent malpractice claims. Of 1285 malpractice claims reported in our review, equipment failure played a role in a median of 15.5% of cases [3].
Error studies specific to ENT surgery
From malpractice studies outside the UK, Tokuda et al. from Japan showed that, of 274 closed claims for medical injury, ENT ranked sixth out of 24 medical and surgical specialities [8] and Reilly et al. in a recently published analysis of all hearing-loss law-suits in the USA over a ten-year period, showed that ENT surgeons were the most commonly sued with an average pay-out of $579,000 [9]. Two other survey-based studies of ENT surgeons across the UK reveal that 9–20% of ENT surgeons may have been involved in wrong-site surgery at some point. The evidence, though sparse and limited indicates that in these countries at least, ENT may be a field where error can be important to identify and mitigate.
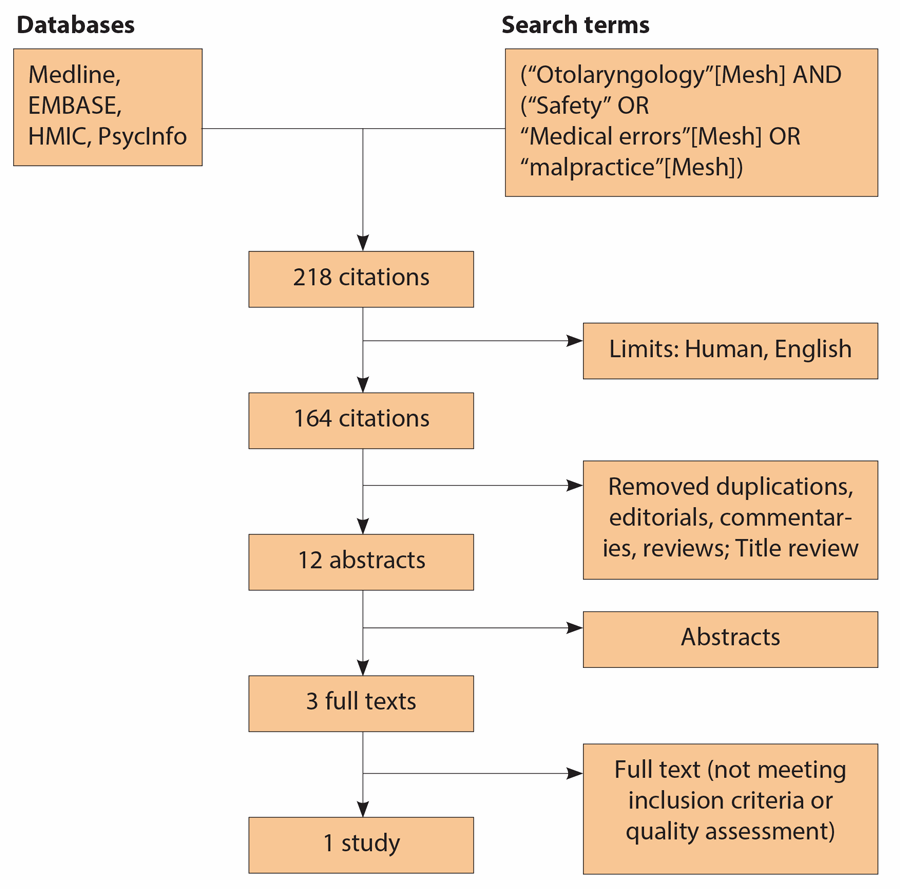
Figure 2: Consort diagram for search strategy, adapted to ENT.
[Last search performed on November 3rd, 2013.]
The published data for safety culture and error in otolaryngology, including in the intra-operative environment is limited; this is particularly true for those dedicated studies where errors are systematically observed. In relation to studies looking at the role of equipment / technology, we modified our search strategy to consider all ENT-related studies and only one met our inclusion criteria (Figure 2) [6]. Here, Shah et al., from a retrospective self-reported study (survey) of 466 ENT surgeons across the UK, constructed a detailed catalogue of 212 reported errors. 9.4% were equipment related (approximately a third due to availability, the rest due to direct failure or incorrect configuration); more significantly, circa 30% of reported equipment problems resulted in morbidity (also termed ‘major’ error) [6]. They also identified three recurrent types of equipment failure: lack of equipment availability in the OR, burns from poorly insulated or incorrectly assembled cautery units and failure of complex equipment [e.g. image guidance system for endoscopic sinus surgery (ESS), coblation unit, nerve integrity monitor] [6].
What we can do – interventions, checklists and team training
Interventions including preoperative checklists, perioperative briefings and staff training have been shown to be effective in decreasing error rates. These measures were shown to reduce error rates by almost 50%, with the most benefit from preoperative checklists [3]. Surgical checklists are now a routine and in many instances, a mandatory part of modern surgical practice. The World Health Organization (WHO) surgical safety checklist developed from the WHO ‘Safe surgery saves lives’ campaign, has been adopted throughout hospitals worldwide and has been shown to be effective in reducing patient harm [7].
As the evidence suggests that the majority of equipment errors are attributable to availability and configuration problems [3], this would suggest that preoperative checklists hold the key for minimising these risks and constitute a tractable component of improving patient safety. Our research in vascular surgery to date indicates that more technologically-intensive procedures, such as those found in ENT surgery, may benefit from pre-procedural rehearsal, which would also provide an opportunity for team members to familiarise themselves with specialist equipment and technology that often only the operator has been able to keep apace with [4, 5], as well as the creation of speciality or procedure-specific checklists that prompt verification of specialist equipment availability and configuration or planning sessions that take place earlier than the day of the procedure. Finally, there must be a systems approach to take into account human factors and human / technology interactions. Training sessions should be used to facilitate the inclusion of technology into the operating environment without increasing error rates. Modifying systems to minimise intrinsic latent errors that propagate specific active human errors stems from the ‘trajectory of accident opportunity’.
In the UK Landscape of Error in Aortic Procedures (LEAP) study, we are assessing error patterns at multiple UK hospitals and their impact on safety and procedural flow on a wider basis to provide an understanding of the entire spectrum of error, including failures and errors that do not necessarily lead directly to patient harm on the recorded occasion, but which increase the risk of harm across the board. Identification of error to guide appropriate intervention is the key to improving the safety of patients. A similar drive in ENT would seem warranted if not already underway.
Acknowledgement
We should like to thank the authors of our original study, Surgical technology and operating-room safety failures: a systematic review of quantitative studies. BMJ quality & safety 2013;22(9):710–8; Rachael Lear (Research Fellow), Celia Riga (Lecturer / Vascular Surgeon), Mohammed S Hamady (Senior Lecturer / Consultant Interventional Radiologist), Krishna Moorthy (Senior Lecturer / Consultant General Surgeon), Ara W Darzi (Professor of Surgery), Nicholas J Cheshire (Professor of Vascular Surgery) and Charles Vincent (Emeritus Professor of Clinical Safety Research & head of Clinical Safety Research Unit).
References
1. Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372:139–44.
2. De Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Quality & Safety in Health Care 2008;17:216–23.
3. Weerakkody RA, Cheshire NJ, Riga C, Lear R, Hamady MS, Moorthy K, Bicknell CD. Surgical technology and operating-room safety failures: a systematic review of quantitative studies. BMJ Quality & Safety 2013;22(9):710–8.
4. Albayati MA, Gohel MS, Patel SR, et al. Idenification of Patient Safety Improvement Targets in Successful Vascular and Endovascular Procedures: Analysis of 251 hours of Complex Arterial Surgery. Eur J Vasc Endovasc Surg 2011;41(6):8.
5. Patel SR, Gohel MS, Hamady M, et al. Reducing errors in combined open/endovascular arterial procedures: influence of a structured mental rehearsal before the endovascular phase. J Endovasc Ther 2012;19:383-9.
6. Shah RK, Kentala E, Healy GB. Classification and consequences of errors in otolaryngology. Laryngoscope 2004;114:1322–35.
7. Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat A-H S, Dellinger EP, Gawande AA. A Surgical Safety Checklist to Reduce Morbidity and Mortality in a Global Population. New Engl J Med 2009;360(5):491–9.
8. Tokuda Y, Kishida N, Konishi R, Koizumi S. Cognitive error as the most frequent contributory factor in cases of medical injury: a study on verdict’s judgment among closed claims in Japan. J Hosp Med 2011;6:109–14.
9. Reilly BK, Horn GM, Sewell RK. Hearing loss resulting in malpractice litigation: what physicians need to know. Laryngoscope 2013;123:112–7.
10. Shah RK, Nussenbaum B, Kienstra M, Glenn M, Brereton J, Patel MM, Roberson DW. Wrong-site sinus surgery in otolaryngology. Otolaryng Head Neck 2010;143:37–41.
11. Shah RK, Arjmand E, Roberson DW, Deutsch E, Derkay C. Variation in surgical time-out and site marking within pediatric otolaryngology. Arch Otolaryng Head Neck 2011;137:69–73.
Declaration of Competing Interests
The research was funded / supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.






