You’ve got an amazing idea for a new device. It is going to change how your speciality of surgery is practised. It will lead to better operative results and lower risks to patients – that’s amazing, can I see it? Here is where the problem starts – how do you take your world-beating, practice-changing idea from concept to market? Professor Rahul Kanegaonkar discusses the facets involved in achieving this aim.
In the summer of 1988, having completed my A-levels, I found myself in the unenviable position of having neither secured a place at university, nor a job. I therefore ‘signed on’ at the local JobCentre Plus. The staff were determined to find me employment and I was interviewed and deemed unfit for sweeping up hair between clients in a local hairdresser and arranging tractor tyres in a warehouse.
I was eventually appointed at Portex Limited. My role in the innovation department involved performing validation studies (e.g. high-volume low-pressure cuffs used on tracheostomy tubes) and, more interestingly, building working prototypes of ideas submitted to the company. Whilst working on a bacterial/viral filter to protect anaesthetic circuits (HIV had necessitated this), I proposed a different configuration that was both smaller and cheaper to manufacture and I was granted my first patent [1].
I have since been involved in dozens of medical innovation projects that some readers may be familiar with and others may not. Some may seem obvious, others fanciful, such as a spectacle display that provides real-time subtitling for patients with hearing loss (https://patents.google.com/patent/US20140236594).
What is medical innovation?
A new process, product, service, method or technology may be considered to be ‘innovative’ and, when applied to healthcare, a ‘medical Innovation’. Hospital Trusts are acutely aware of the need to innovate, and conscious of the potential cost saving of a successful process and financial return of a successful product.
The innovation process
The key is to understand and navigate the pathway required to successfully bring an Innovation to market. This is outlined in Figure 1.
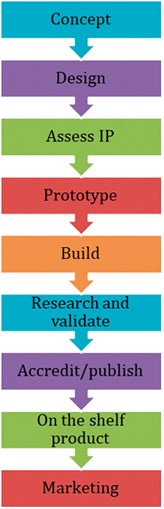
Figure 1. The innovation pathway.
Concept
Successful projects arise from first identifying a clinical need, matching this with an observation and then acting upon it. With an interest in balance disorders, I was disappointed to learn that our departmental force plate was beyond repair. Viv Austin, a Digital Architect and I, developed an iPhone application, ‘D+R Balance’, that records rotation on Fukuda step testing and postural sway on Romberg’s Testing. The app required appropriate validation and CE approval via the MHRA [2,3].
Tamsin Brown’s ‘Hear Glue Ear’ bone conductor and app are timely to address the delay in intervention for patients with middle ear effusions.
Design
A good design should be simple and clearly resolve the clinical problem that it addresses. Complex designs or designs that set out to solve multiple problems should be avoided if possible.
Assess intellectual property (IP)
Is your idea truly novel? It is worthwhile spending time researching patents, publications and the web. It is often useful to hire a specialist in this field as some patents may be difficult to find.
Prototype
I always recommend that innovators fashion prototypes themselves. This allows them to gain an understanding of the materials that may be used, and the parts required to develop a working model. For example, a piezoelectric plate soldered to a 3.5mm audio jack will provide both an acoustic and bone conducted stimulus. The iPhone app we developed, D+R Hearing, replaced standard tuning fork testing and assessed bone conductor thresholds. Unfortunately, a smartphone shift to Bluetooth headsets made this project unfeasible and it was therefore suspended.
Build
It is often the case that a physical build will require working with an established company. One should always seek a non-disclosure agreement (NDA) when approaching potential collaborators and I would encourage innovators to keep their ideas private and not to present them at meetings nor publish them on the internet.
Some projects can be built with a good, determined team. For example, Docbook.co.uk was an online picture book series that Dr Aslan Mirza (a GP with formidable IT skills), Dr Junaid Bajwa (now Chief Medical Scientist for Microsoft) and I published in 2007. This online picture book series attracted over 3.2 million hits from over 150 countries.
More complex digital platforms require an understanding of security and regional protocols. We recently developed a physiotherapy platform, ‘D+R Therapy’ that allows remote supervision of patients by exploiting the patient’s own smartphone. This not only improves compliance but also reduces the potential need for weekly review [4,5]. The cost saving to the NHS is potentially enormous.
Research and validation
Some, but not all, innovation projects will require a form of validation. The ‘Cupris iPhone Otoscope’ for example, developed by Mr Julian Hamann, provides an elegant remote method for assessing and recording ear pathology that was validated by our team. A simple lens attachment converts the smartphone into a digital otoscope that can be used as a screening tool for potential referrals.
Accredit/publish
Clinicians are, by nature, risk averse. Many, myself included, would be more comfortable using a new innovation having assessed the evidence base. Hence, peer reviewed publication is, on occasion, a necessity.
On the shelf product and marketing
Marketing of a novel innovation can require substantial investment. Advertising in medical journals and via specialist marketing companies may be essential to get the word out.
The landscape for medical innovation has expanded over recent years. Several platforms have been launched, offering guidance. Innovators must have a clear understanding of the role of the platform, the services they provide and their cost, both in the initial phase and downstream.
The Clinical Entrepreneurship Programme, launched by Professor Tony Young, the National Clinical Director for Innovation at NHS England, is an extraordinary platform that provides tuition and networking opportunities. Successful applicants are exposed to tutorials that cover such topics as how to pitch, marketing and fund raising.
Conclusion
I would certainly encourage clinicians at all stages of their careers to consider developing their medical innovation projects. Some projects may fail, and I often remind innovators that their first innovation may not be their best, but that they can understand the process and avoid pitfalls in future projects. A minority may prove lucrative, but they will all be interesting and challenging.
References
1. Kanegaonkar RG. Radial Bacterial/Viral Filter. UK Patent No. GB 2 233 904 B (1991).
2. Whittaker M, Mathew A, Kanani R, Kanegaonkar RG. Assessing the Unterberger test: introduction of a novel smartphone application. J Laryngol Otol 2014;128(11):958-60.
3. Yvon C, Najuko-Mafemera A, Kanegaonkar R. The D+R Balance application: a novel method of assessing postural sway. J Laryngol Otol 2015;129(8):773-8.
4. Vaish A, Ahmed S, Shetty A. Remote physiotherapy monitoring using the novel D+R Therapy iPhone application. J Clin Orthop Trauma 2017;8(1):21-4.
5. Saleh A, Parkar F, Tolat A. The Use of the D+R Therapy Physiotherapy iPhone Application in the Management of Radial Head Fracture. Res Rev Orthop 2018;2(1).
Declaration of Competing Interests: The author is a Director of D+R Medical Limited that developed the D+R Therapy Platform.




