Should we be using new or novel objective measures and imaging to assist with our cochlear implant patients? Debi Vickers and Shak Saeed describe current clinical techniques and present advances that have the potential to optimise outcomes.
Introduction
It is well known that there are a range of outcomes for cochlear implant users. Many factors contribute to this diversity, some of which are hard for clinicians to influence such as cognitive processing abilities, home language and family engagement. Other factors, if known about, may inform clinical practice and potentially improve performance. Such factors relate to the viability of the electrode-neuron interface [1], affected by insertion trauma, electrodes in the incorrect scala, electrode distance to modiolus and neural survival.
For some individuals the difficulties may arise higher up in the auditory system, for example the extent of cortical re-organisation could affect performance. In this era of personalised medicine optimising the implant for each individual is crucial and with the use of objective measures and imaging clinicians have tools to support intervention.
Pre-implantation
Objective measures of hearing acuity are a valuable audiological tool because they do not require a patient response. In the UK and many other countries prior to implantation babies and infants will have undergone multiple objective tests of hearing such as otoacoustic emissions (OAEs), auditory brainstem responses (ABRs), auditory steady state responses (ASSR) and/or cortical responses to speech. The majority of patients, will typically have a preoperative magnetic resonance imaging (MRI) and/or computed tomography (CT) scan to assess cochlear presence, morphology and patency, presence and calibre of the auditory nerve all of which can assist the surgeon in electrode array selection and the insertion technique.
In some clinical cases the implant team require confidence that the auditory system will respond to the electrical stimulation before implantation. One approach that was more in favour two decades ago was that of electrocochleography (eCochG) using a transtympanic electrode placed on the promontory. The technique detects the cochlear microphonic, an indicator of hair cell function and also the auditory nerve neurophonic to show neural sensitivity.
This approach fell out of favour as MRI techniques advanced to visualise the auditory nerve and also because responses were not reliable. Some implant programmes prefer to use electrical ABRs (eABR) with the transtympanic ‘golf club’ electrodes because responses are more consistent. This is especially useful in cases of auditory neuropathy spectrum disorder, hypoplastic cochlear nerves and certain neurological conditions. Nowadays, because many implant recipients have residual hearing there has been a move to utilise the eCochG in a different way, taking advantage of the telemetry capabilities of the implant electrodes to record responses during electrode insertion [2]. This technique has the potential to monitor the function of hair cells and neurones during implantation to reduce trauma.
Post-implantation
Imaging
Typically after implantation a modified Stenver’s view X-ray is taken to check electrode placement. In some countries postoperative CT scans are available providing detail about scalar position and distance to the modiolus for individual electrode contacts. This level of detail has been used to guide mapping with positive outcomes for some individuals [3]. Some health systems do not permit the additional postoperative CT scans because of concerns around the added radiation dose. Cone beam CT is a potential alternative due to lower radiation dose, reduced metal artefact and enhanced detail [4] (Figure 1).
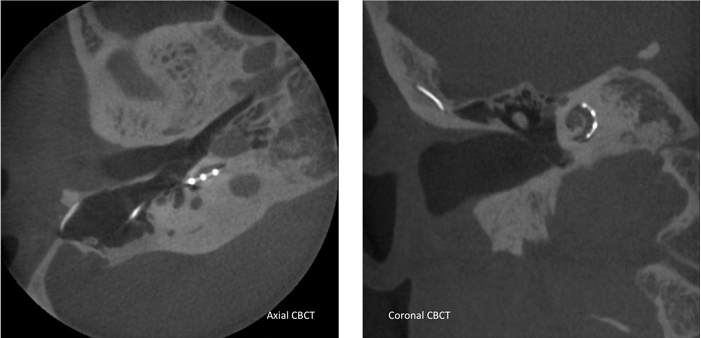
Figure 1. Left: Axial cone beam CT confirming that the array is in the scala tympani in the section shown. Right: Coronal cone beam CT showing the electrode array coursing around the lateral scalar wall.
Imaging the cochlea provides useful information but it does not show neural survival and functionality throughout the auditory pathway. This is best assessed with objective measures once the implant is in place.
Neural responses
There are two main approaches for objectively monitoring auditory nerve response following implantation, these are the electrically evoked compound action potential (eCAP) and the electrically evoked stapedial reflex response (eSRT). These responses can be used for determining fitting levels for patients who cannot reliably respond. Neither approach perfectly predicts levels but they provide estimates of a region where psychophysical levels should be set. When the responses are present the predictions are good at providing access to sound without it being too loud. The eCAP measurements implemented in clinical software can be used to measure spread of excitation (SoE).
This shows how far the excitation spreads when an electrode is stimulated, potentially masking responses to information at other electrode sites, thus providing an indication of peripheral spectral resolution, known to be related to speech perception abilities. The SoE measure can be used to highlight if particular electrodes provide greater masking or have unusual masking patterns that could be indicative of tip foldover or cross-turn stimulation [5].
Brainstem responses
The eABR (wave V) can also be used to estimate psychophysical fitting levels, although it has the disadvantage that the technique is more complex, but the responses are reliable. In addition, because the eABR is measured at a higher stage in the auditory pathway, it can provide more information about sound processing. An example of this relates to advanced bilateral fitting. One of the issues is that there is an inter-aural place mismatch due to different electrode placements and neural survival resulting in different frequencies stimulating different regions in each cochlear. It is predicted that if the information supplied to each ear can be matched, to some extent, the brain will be able to combine interaural cues to achieve binaural hearing.
To this end the binaural interaction component (BIC) can be measured. The BIC is the amplitude of the eABR wave V measured during bilateral stimulation subtracted from the summed wave V amplitudes for monaural stimulation (Figure 2). The amplitude of the BIC reduces as the electrode separation increases, suggesting that the greater the mismatch the smaller the BIC. Hu and Dietz (2015) measured the BIC in response to interaural channel stimulation and found that the BIC amplitude correlated well with perception of interaural timing differences [6].
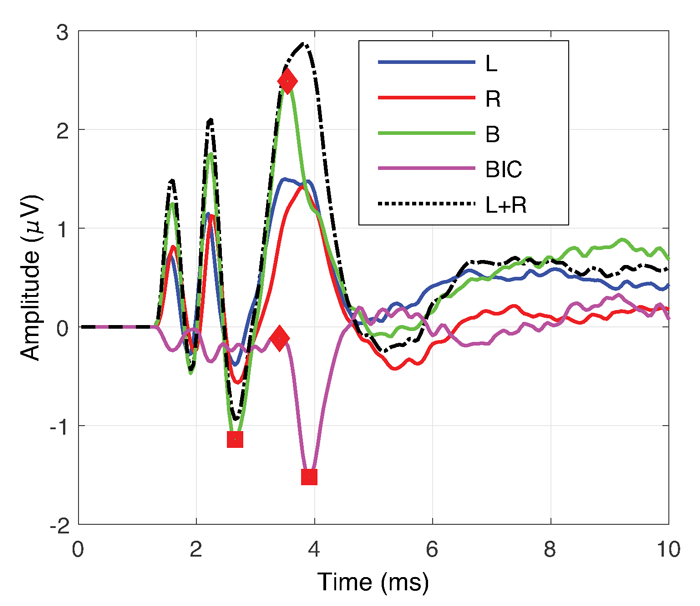
Figure 2. Shows the traces for determining the BIC. The blue trace is the eABR response for left (L) ear alone. The red trace is for the right (R) ear. The green is the response when both ears (B) are stimulated together. The black dashed line is the summed response for left and right ears. The pink trace shows the BIC (BIC=B-(L+R)).
Cortical responses
Cortical responses are able to show how sound is processed at higher levels in the auditory system and are good for observing neuro-plastic changes occurring following activation. The time course for changes is not fully understood but it is certainly different across individuals. The latency and amplitude of the P1 peak in the cortical response can be used as a developmental biomarker for the postimplantation maturation of the auditory system in children. Sharma and Dorman (2006) used this response to define critical periods for age at implantation in children and showed that appropriate maturation is more likely when implanted before 3.5 years and that the sensitive period ends around seven years when the cortical reorganisation becomes far more limited [7]. With such measures the maturation process can be monitored post-implantation and intervention provided accordingly.
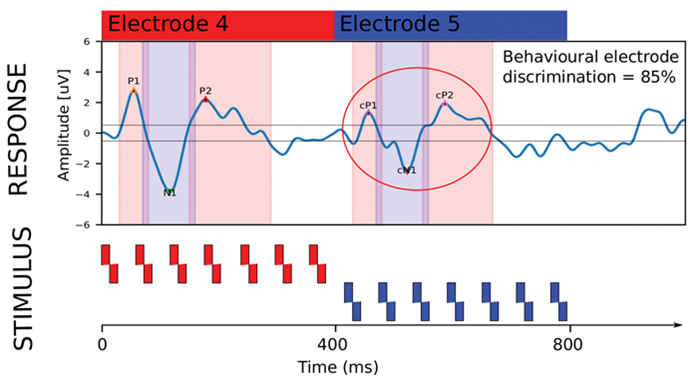
Figure 3. Demonstrating the eACC cortical response to a change in electrode stimulation. For the first 400 ms electrode 4 was stimulated and a clear P1-N1-P2 onset response is seen. At 400 ms electrode 5 is stimulated and the eACC is shown in response to the change. For this individual the change is detected in the eACC and behaviourally they were able to discriminate the electrodes as well.
There are many cortical response measurement techniques and one that is currently of great interest is the electrically evoked auditory change complex (eACC). The eACC can be used for estimating discrimination abilities objectively and is a response to a change in an ongoing stimulus. One area of success has been in measuring electrode discrimination; see Figure 3 for a diagram showing the eACC response to a change in electrode stimulation. Pass/fail criteria for electrode discrimination based on eACC responses are highly correlated with behavioural responses and in turn speech perception abilities [8].
For many individuals (usually pre-lingual adults) the eACC precedes behavioural discrimination. This information could inform rehabilitation or guide mapping.
Summary
This overview has provided examples of current clinical objective measures and imaging techniques and shown advances that have potential for optimising cochlear implant outcomes. It is not exhaustive but broadly covers how objective measures and imaging can help at different stages of implantation to understand sound transmission through the auditory pathway. As yet, guidance on how to utilise the information for surgical techniques, implant mapping and rehabilitation are not well understood. It is a critical area for controlled intervention studies so that sophisticated objective measures and imaging techniques can be effectively utilised for advancing implant performance.
References
1. Bierer J. Probing the electrode-neuron interface with focused cochlear implant stimulation. Trends in Amplification 2010;14:84-95.
2. Bester CW, Campbell L, Dragovic A, Collins A, O’Leary S. Characterizing Electrocochleography in Cochlear Implant Recipients with Residual Low-Frequency Hearing. Frontiers in Neuroscience 2017;11: [Online research article] doi.org/10.3389/fnins.2017.00141.
3. Noble J, Gifford R, Hedley-Williams A, Dawant B, Labadie R. Clinical evaluation of an image guided cochlear implant programming strategy. Audiology and Neurotology 2014;19:400-11.
4. Saeed S, Selvadurai D, Beale T, et al. The use of cone-beam computed tomography to determine cochlear implant electrode position in human temporal bones. Otology Neurotology 2014;35:1338-44.
5. Grolman W, Maat A, Verdam F, et al. Spread of excitation measurements for the detection of electrode array foldovers: a prospective study comparing 3-dimensional rotational x-ray and intraoperative spread of excitation measurements. Otology Neurotology 2009;30:27-33.
6. Hu H, Dietz M. Comparison of Inter-aural Electrode Pairing Methods for Bilateral Cochlear Implants. Trends in Hearing 2015;19:1-22
7. Sharma A, Dorman MF. Central auditory development in children with cochlear implants: Clinical implications. Advances in Oto-Rhino-Laryngology 2006;64:66-88.
8. Mathew R, Undurraga J, Li G, et al. Objective assessment of electrode discrimination with the auditory change complex in adult cochlear implant users. Hearing Research 2017; [Epub ahead of print] doi.org/10.1016/j.heares.2017.07.008.
Acknowledgements: Thanks to Rajeev Mathew and Hongmei Hu for providing figures and to Anzel Britz for reviewing the text.
Declaration of competing interests: Authors have received small research grants from MED‑EL, Advanced Bionics, Cochlear Corporation and Oticon Medical but the contents of this review relate to devices from all implant companies and no proprietary terms have been used.





