Just before I left Cambridge to work with the Hearing Sciences group in Nottingham, I spent a very happy hour alone in the company of Professor Charles Liberman, the Director of the Eaton-Peabody Laboratories based at the Massachusetts Eye and Ear Infirmary, Boston USA, whose work has led to some of the most important discoveries and insights into auditory function in the last 30 years. The conversation ranged from hyperacusis, to tinnitus, and settled on cochlear synaptopathy. I have often wished for an opportunity to pick that discussion back up, and to share Charlie’s wisdom and experience with the wider hearing community. So, when the possibility of this interview came up, I was delighted!

Professor Charles Liberman.
Charlie, wonderful to talk with you again, thank you for your time. I would be interested to hear how your interest in hearing began.
Hi Dave – I’m glad to participate. I’ve always been interested in music, and, as a freshman in college, I was considering a career as a classical organist. Then I took a course in neurobiology and became fascinated with sensory neuroscience. I decided that the two interests came together in the study of hearing: it seemed feasible to be a scientist and pursue music as a hobby, but probably not reasonable to be a professional musician and try to pursue science as a hobby.
Who were your mentors in your early career?
When I was a junior in college, I wanted to take a course in the neuroscience of hearing, but none were offered in the Boston area. I had the option of taking a ‘reading course’ for credit, but needed to find a faculty member to sponsor me. After some bouncing around, I found Nelson YS Kiang, who had joint appointments at Harvard, MIT and the Massachusetts Eye and Ear Infirmary (MEEI). He became my reading-course sponsor, my undergraduate honours-thesis mentor, my graduate-student advisor and then my first boss at the MEEI as a junior faculty member. Kiang was famous for his keen intellect, and notorious for his brutally frank style of scientific criticism. Those of us who survived his tutelage, learned a lot about hearing but, more importantly, we learned how to dissect scientific arguments, and how to think rigorously about scientific investigation.
“We saw that noise exposures caused dramatic loss of synapses on surviving inner hair cells, even in ears where thresholds had completely recovered.”
What do you perceive to be your earliest important research finding?
My thesis project was on the effects of acoustic overexposure on responses of single fibres in the auditory nerve. To quantify noise-induced threshold shifts, I had to define ‘normal’ thresholds in our animal model (cat). That was complicated, because our ‘normals’ always showed a mixture of low- and high-threshold fibres. Prior researchers assumed that the high-threshold fibres were pathological, because our cats were strays from shelters (about to be euthanised) with an unknown history of disease and noise exposure. To clarify whether this threshold heterogeneity was a bug or a feature, I raised several litters of cats in the laboratory, in a controlled environment, where the loudest sounds were their own vocalisations. I showed that the high-threshold fibres were still present in these quiet-raised ears and that there was a very strict relation between threshold sensitivity and spontaneous rate (SR), i.e. the rate of action potentials seen in complete silence). The division I suggested between high-, medium- and low-SR fibres survives to this day. It is important because high-threshold fibres (with the lowest SRs) extend the dynamic range of the auditory periphery and help us hear in a noisy environment. These ideas became even more relevant when we discovered cochlear synaptopathy and the fact that low-SR fibres are the first to degenerate.
You were instrumental in the identification of cochlear synaptopathy (hidden hearing loss): please could you briefly define that, and how the discovery came about?
Sound-evoked signals are carried from inner hair cells to the brain by fibres of the auditory nerve. Each inner hair cell is normally contacted by ~20 auditory nerve fibres, with representatives from each of the SR groups described above. Cochlear synaptopathy refers to a condition in which surviving inner hair cells have been disconnected from some of the auditory nerve fibres that used to contact them. Threshold measures, including the audiogram, are not sensitive to loss of neurons, especially the high-threshold neurons, so long as the hair cells remain intact. Therefore, a lot of cochlear synaptopathy can ‘hide’ behind the audiogram, though it may compromise our ability to understand complex signals in poor listening environments. Cochlear synaptopathy is also ‘hidden’ because it is only the synaptic boutons and peripheral axons of the auditory nerve fibres that die (see Figure 1), at least initially. The (spiral ganglion) cell bodies and central axons can survive for decades. Whereas the hair cells and spiral ganglion cells are easy to see in the light microscope, the synaptic contacts are more difficult to visualise.
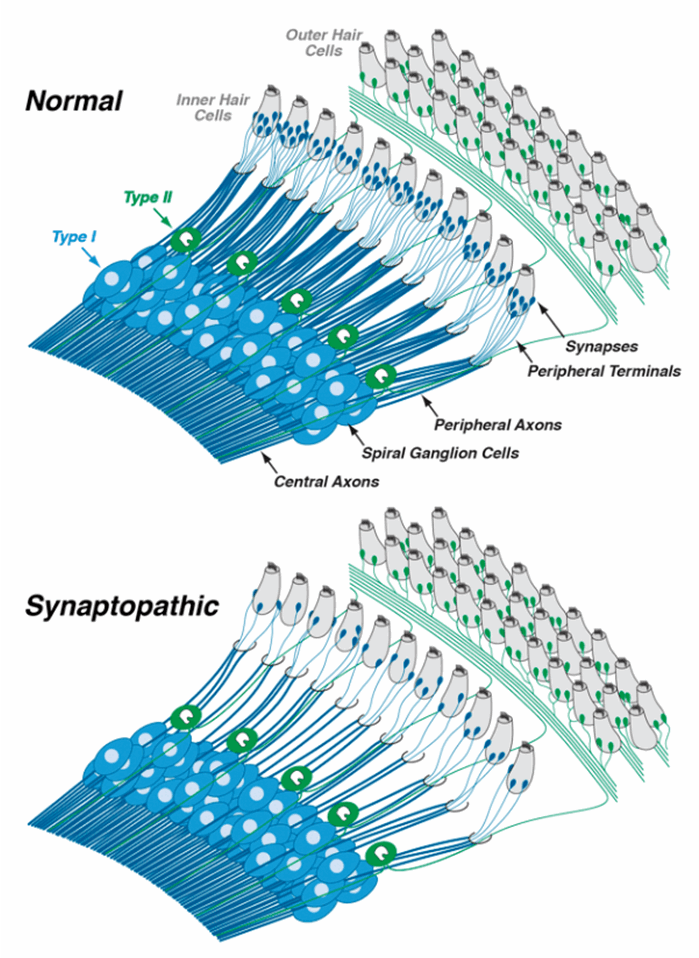
Figure 1. In the normal ear, each inner hair cell sends signals to the brain via numerous type-I spiral ganglion neurons (top schematic). In the synaptopathic ear (bottom schematic) many of the synaptic contacts between type-I neurons and inner hair cells have degenerated, thus many of the surviving spiral ganglion cells are silenced. The type-II spiral ganglion neurons innervating outer hair cells are unaffected, but the signals they carry likely have more to do with pain sensation than with hearing per se. Drawings are adapted from Liberman [1].
In the early 1980s, I (and others) showed that, immediately after acoustic overstimulation, peripheral terminals of auditory-nerve fibres were swollen at their synaptic contacts with inner hair cells. This swelling disappeared within a few days, whether or not thresholds returned to normal. Many assumed that these swollen fibres must have recovered if the threshold recovered. I considered it an open question, since I knew that neural degeneration could hide behind the audiogram.
“I’m confident that effective treatments for numerous kinds of sensorineural hearing loss are now on the horizon.”
The question came again to the fore when Sharon Kujawa, my colleague at the MEEI, asked me to help her assess the histopathology in experiments probing whether noise-exposed ears age differently from unexposed ears. In ears examined months to years after exposure, we noted that spiral ganglion cells were degenerating, even though the hair cells were not. That violated the long-standing dogma that the auditory nerve degenerated if, and only if, inner hair cells were destroyed, and it suggested that those swollen terminals seen immediately after exposure might have been signs of irreversible damage. To answer the question, we took advantage of the advances in immunohistochemistry that allowed to use antibodies to stain specific synaptic proteins and thereby count synapses in the light microscope. As soon as we got the new staining techniques working, we saw that noise exposures caused dramatic loss of synapses on surviving inner hair cells, even in ears where thresholds had completely recovered.
Are there potentially important clinical implications to your work on cochlear synaptopathy?
Yes, we have autopsy material from ageing humans showing that, as in animals, many surviving inner hair cells have been partially disconnected from the auditory nerve. Each disconnected spiral ganglion cell is completely silenced and that loss of activity decreases the fidelity of the signals transmitted to the brain. We believe this silencing of neural connections compromises hearing abilities in tasks such as word recognition in noise. Importantly, in synaptopathic ears, the spiral ganglion cells and their central axons survive for decades, while only the peripheral axon degenerates. These peripheral axons can be regenerated, even in adult animals, by local cochlear delivery of neurotrophins, the naturally occurring proteins released by inner ear supporting cells that help keep auditory nerve fibres alive. We hope that these approaches can eventually be applied to humans to improve speech discrimination abilities, especially in age-related hearing loss.
Your work has also proposed that cochlear synaptopathy may be involved in tinnitus and hyperacusis [2]. Have you developed those ideas at all?
Since acoustic overexposure is the most reliable way to produce tinnitus and hyperacusis in humans, and since both perceptual anomalies can occur without audiometric shifts, and since auditory-nerve synapses are the most vulnerable elements in noise damage, it was natural to hypothesise that synaptopathy might be a key elicitor of tinnitus and hyperacusis. There is now evidence from animal models that this type of peripheral neural loss is transformed into hyperactivity (both spontaneous and sound-evoked) throughout the central auditory pathways, as central neuronal circuits rebalance the excitatory and inhibitory inputs in response to decreasing ascending signals. It’s intriguing that, in some cochlear implant users, simply turning on the implant, and thereby restoring spontaneous activity to heretofore silent auditory nerve fibres, can attenuate the tinnitus percept. It suggests that reconnecting silenced spiral ganglion neurons to hair cells could also be a treatment for tinnitus. It might also be a cure for some types of hyperacusis, but see below.
Staying with hyperacusis, as it is the subject of this section, the identification of nociceptive (pain-signalling) fibres in the cochlear nerve [3] has been of major interest to those who experience, treat, or research ‘pain hyperacusis’. I’d love to hear your present thoughts on that.
Auditory neuroscientists have long speculated that the sensory fibres connecting outer hair cells to the brain, i.e. the unmyelinated type-II neurons (see Figure 1), were pain fibres based on analogy to the somatosensory system, where pain sensations are carried by small unmyelinated fibres. Recent studies suggest that, indeed, these neurons only respond when there is damage to the hair cells. If true, and if hyperactivity of these type-II neurons is the basis for pain hyperacusis, it might be possible to design a drug therapy that selectively blocks signalling in the type-II system without blocking signalling in the type-I fibres that we need for hearing. A challenge in pursuing these ideas is to develop an animal model of pain hyperacusis. I believe that pain hyperacusis, which can be very long-lasting, is fundamentally different from annoyance hyperacusis, where the discomfort ends when the stimulus ends, and it will be difficult to distinguish these two types of hyperacusis in an animal model.
What topics in hearing research are exciting you at the moment?
This is a very exciting time for hearing research. Biotech companies are springing up right and left, because we, as a field, are beginning to understand enough about the cellular mechanisms and molecular signalling pathways underlying degeneration and regeneration in the inner ear that development of rational therapeutics has begun. The inner ear is amenable to local delivery of drugs and/or viruses carrying gene cargo, so I’m confident that effective treatments for numerous kinds of sensorineural hearing loss are now on the horizon.
Your work amalgamates insights from both basic auditory neuroscience, and issues of major clinical importance: how have you achieved and sustained that fusion?
The key has been to do basic science in a clinical environment. The Eaton-Peabody Laboratories, where I have worked since I was a graduate student, are located within an active clinical facility, the Massachusetts Eye and Ear. Here, basic scientists like me mingle with ENT clinicians and audiologists, so we are constantly reminded of the important clinical issues. I was attracted to science because I love solving puzzles. Since many puzzles can be equally challenging, I always chose to work on problems that might matter to patients sooner rather than later.
Is there advice that you would give to a young hearing researcher, from either a clinical or science discipline, just embarking on a career in hearing research?
If you really love scientific inquiry, it’s a great time to enter the hearing field. It will always be a bit of a struggle to succeed in academia, as the availability of federal funding waxes and wanes inversely with the federal deficit. However, the entrance of biotech into the hearing space provides many more career options (and is in the process of raising salaries across the board). With respect to undergraduate education, I recommend taking the most quantitative approach that you can handle, e.g. biomedical engineering rather than biology. I was a biology major, and used to be irritated when my mentor said that it was easier for an engineer to learn biology than for a biologist to learn engineering, but he was right. And an engineering background teaches you rigorous problem-solving approaches that can be applied to many types of problems.
“I always have several Facebook Scrabble games going with friends and relatives, and I almost always do the Sunday crossword in the New York Times.”
In the life of a researcher there is always something that needs doing: writing a grant, a paper, or a talk, reviewing the work of others, or reading within and around your subject. How do you relax when you get the chance?
Music has always served as a terrific way to unwind (it was easy to keep it as a hobby). I continue to play piano (blues, ragtime) and guitar (bluegrass, folk, popular, and blues). I enjoy scuba and skiing, I always have several Facebook Scrabble games going with friends and relatives, and I almost always do the Sunday crossword in the New York Times.
References
1. Liberman MC. Noise-induced and age-related hearing loss: new perspectives and potential therapies. Faculty of 1000 Research 2017;6:927.
2. Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol 2014;111(3):552-64.
3. Flores EN, Duggan A, Madathany T. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol 2015 2;25(5):606-12.






