Patrik Westerkull (PW), Otorix AB, and Ann-Louise McDermott (A-LM), ENT Consultant at Birmingham Children’s Hospital, tell us about the Adjoin bone conduction device, a non-surgical bone-conduction option developed by Otorix. They explain how the product works, the background to the project and why this is an important new treatment option, particularly for children and young people with a hearing loss.

Patrik Westerkull.

Ann-Louise McDermott.
In a nutshell, can you explain the Adjoin treatment concept?
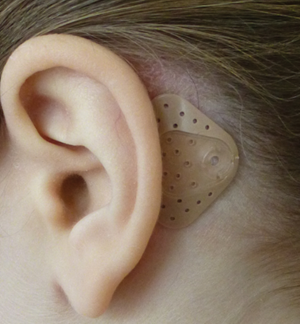
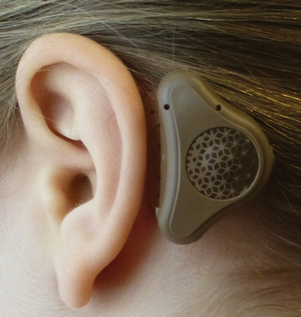
PW: The Adjoin system is a non-surgical bone conduction system with a processor that is connected to an adhesive attachment adhered to the hairless skin behind the pinna. The sound processor is a further development of the technology used in bone anchored hearing aids. The sound processor can be easily snapped on and off from the adhesive attachment during the day in a similar way to how a bone anchored hearing aid is connected to a percutaneous implant. The adhesive attachment is worn day and night and it is typically worn for three to seven days before it is removed and replaced with a new one.
What is your involvement in Adjoin and how did you get involved?
A-LM: Patrik approached the Bone Anchored Hearing Implant team at Birmingham Children’s Hospital two years ago with the novel idea of an adhesive adapter to facilitate the sound processor without the need for surgery or a headband. Many older children refuse any trial of a bone conduction hearing device because they are “put off” or dissuaded by the appearance and discomfort of the alternative headbands, softbands and spectacle options. The possibility of a discrete adhesive adapter to allow the child to trial this form of hearing device was an exciting concept that we were keen to be involved with.
Tell us about the background to developing this concept. What is the story behind Adjoin?
PW: Having worked on the development of bone anchored hearing aids for more than 20 years, I have seen the need for a non-surgical bone conductor that, at least for conductive hearing losses, could perform in a similar way to a bone anchored hearing aid. The drawbacks of conventional bone conductors, particularly poor wearing comfort, unreliable retention and poor aesthetics, have thus far mainly been addressed by implantable devices such as the bone anchored hearing aid and the Bonebridge device. The need for surgery adds of course additional complexity to the whole treatment. Some patients are unsuitable for or unwilling to undergo implant surgery or to have a percutaneous retention or implanted magnets. To date, acceptable non-surgical alternatives have been lacking.
I had a vision to develop an adhesive bone conduction system that included no pressure against the skin, and this idea challenged several established dogmas. All previous bone conductors transmitting vibrations through the skin involved pressure on the skin from the device, and this principle was seen as inevitable. Most people also did not believe it would be possible to develop an adhesive concept that could be repeatedly attached and removed without irritating the skin and still offer secure retention of the weight of a sound processor worn in daily life. Understanding patient needs, applying multidisciplinary knowledge in physics and having the courage to challenge the established solutions and dogmas made this development possible, and the rest was regular hard work.
Physics such as mechanical transmission dynamics, the fact that skin mainly consists of water which is an incompressible fluid, and findings about the efficiency of different stimulation positions on the head can offer guidance to understanding how this works and how efficient hearing stimulation can be achieved with this concept. When it comes to the development of the unique adhesive concept, it was indeed a project that required both innovative solutions and collaboration with world leading experts in the field of medical adhesives and skin interaction. The efforts paid off and we have today a unique new patented system that we feel is a new era in bone conduction. Otorix AB is an independent research company that I founded in 2004, and the idea then was to develop an improved bone anchored hearing aid, the result of which was the Ponto system that was sold to Oticon in 2006. After supporting the development and launch of this, it was time for me to see if it was possible to solve some of the clinical challenges in the field through further completely new solutions. A new idea was born, and here we are. The Otorix activities with the Adjoin development and evaluation have been independent from Oticon or any other company.
How is this treatment option different to existing bone conduction treatments?
PW: Compared to other bone conductors the adjoin system does not require any surgery and this as a key aspect for many users and clinicians, and since it does not include any headband arrangements it very easy and attractive to fit. The processor is easily connected to the adhesive attachment. Initially we evaluated the principle of the concept by using the patient’s existing sound processors. In this case, most of the patients were already using the Oticon Medical Ponto sound processor with a softband and because of this we made a modified adhesive attachment so that this device could be used on our adhesive concept before we had developed our own processor.
Optimising a sound processor for the Adjoin application was important in developing an efficient new system. Our new sound processor includes improved vibrator technology and sound processing as well as significant anatomical and practical design improvements compared to regular bone anchored hearing aids.
A-LM: The Oticon Medical Ponto sound processor that we have initially used with the adjoin adapter is a well-established and excellent hearing device that is currently worn on either a headband or directly on a percutaneous surgically implanted abutment.
The Adjoin treatment option is different to existing bone conduction treatments by simply allowing the child to wear a similar hearing device on an adhesive adapter without the need for any surgical intervention or use of any headband. It can be placed directly over the mastoid area and is positioned nearer to the cochlea. As a result, it is very discrete and very acceptable to young children and adolescents who are developing self-awareness, and at the same time provides good audiological results.
Why is it important to have a non-surgical alternative to bone conduction surgery?
A-LM: A conductive hearing loss is common in childhood so a nonsurgical bone conduction solution is essential, All surgery carries inherent risks and bone conduction surgery is no exception. Percutaneous implants have a risk of local skin irritation, infection and there is a risk of implant loss which is a recognised problem in paediatrics. To have a non-surgical option is a huge advantage when working with children with permanent and long-standing hearing loss, especially when it is so cosmetically acceptable and provides good audiological results. In our experience , the adhesive method of wearing a bone conduction device has allowed us to treat the hearing loss of many children who would otherwise have refused any intervention, both headband or surgical. For those children alone, the Adjoin adhesive adapter and sound processor has made a significant impact to their lives and their education.
How does it work?
PW: Adjoin works without any pressure against the skin and the sound quality is still as good as with existing bone anchored hearing aids connected to softband or headband arrangements. The main advantages with Adjoin are that the wearing comfort is excellent, the sound quality is very good, the device is well retained without any bulky arrangements and no surgery is required.
Adjoin adhesive bone conduction attachment.
Adjoin bone conduction sound processor.
Can it be used in single sided deafness?
A-LM: It can be used for single sided deafness but to get the best audiological results, a more powerful sound processor is necessary and such a device is heavier. This may result in more frequent displacement of the adhesive adapter and feedback may also be an issue.
PW: We have not conducted any studies on this patient group at this stage.
How can the adhesive work without irritating the skin?
PW: The secret is in the unique micro and macroscopic design of the adhesive arrangement; how it adheres, how it transports moisture and how it interacts with the skin. An important factor is also that the sound processor can be taken on and off easily without tearing the adhesive from the skin. The change intervals of the adhesive attachment can therefore be optimised to suit the skin and these intervals can be chosen independently from the intervals of taking the sound processor on and off. An important prerequisite for good adhesion is that the adhesive is attached to skin that is dry and hairless. When the attachment is in place it is worn for a couple of days and it can also be worn in, for example, the shower. The time between changing the adhesive attachment is individual and may vary with skin type and activity level etc.
Is there much drop off in the high frequencies because of transcutaneous attenuation?
PW: The Adjoin system is sufficiently powerful for patients with pure conductive losses where the inner ear function is fairly normal or has a very mild sensorineural component, and this limitation is similar for any ear level BC device retained with magnet or band arrangements where the vibrations are transmitted through the skin. Since our new sound processor has more high frequency output it may offer some further improvement, however implant devices where the vibrator is firmly connected to the skull bone will still offer higher gain and higher output, and implant devices that are percutaneous or have an implanted vibrator should always be considered for patients with, for example, mixed hearing losses. Such implant devices are still very important treatment options.
What kind of patient might benefit from this treatment?
A-LM: Any child with a conductive hearing loss and normal cochlear function.
What makes this such an important treatment option?
A-LM: The Adjoin adapter is a very important treatment option predominantly because it is so acceptable to children and adolescents who are very concerned about their appearance and the impressions of their peer groups. It allows a much larger population with hearing loss to accept treatment that they would otherwise have refused. We have witnessed a very positive impact on their quality of life and education. There is always the option to move to surgery at any time however we have found that many of our children are very satisfied with the device on the Adjoin adapter, and are happy to continue for the foreseeable future wearing their bone conduction device in this manner.
What trials have been conducted to date and what were the results?
A-LM: In Birmingham Children’s Hospital, we are currently completing the final assessments for the first 20 children who trialled the adhesive adapter when used with the Oticon Medical Ponto sound processor. The children had very few problems with the adhesive, and the majority only changed the adhesive pad every three days. Only one child had a very mild skin irritation however this child suffered from pre-existing eczema. Finally, the results have shown overwhelming patient satisfaction with the adapter and the audiological outcomes are comparable to the same device when worn on a headband.
PW: There have been clinical and audiometric trials and research in Sweden, the Netherlands and in the UK. The acoustic performance has been investigated both in a research project at the Sahlgren Academy in Gothenburg and at the Radboud University Nijmegen, Medical Centre in the Netherlands. The hearing thresholds were found to be very similar for the Adjoin compared to the band arrangements that included a constant pressure. Although a lot of our initial focus has been on children, there are certainly a lot of adults who could benefit just as much from this concept.
How can we find out more? When will the product be available?
PW: Scientific publications presenting clinical results are underway and it has also been presented at some scientific conferences. The technology is currently being fine-tuned in terms of manufacturing processes and it is our ambition to collaborate with a leading industrial player to bring this to clinics and patients worldwide. We are aiming to make it available to clinics within six to 12 months from now. In conjunction with the market introduction, information material etc. will start to be rolled out, although we are keeping a low profile on this at this point.