
Obstructive sleep apnoea has been treated in many different ways over the years. We hear from Yakubu Karagama about one of the latest surgical developments.
Introduction
Obstructive sleep apnoea (OSA) is by far the most common sleep disorder, affecting all ages and sexes. OSA is defined as a repeated obstruction of the upper airway during sleep. It is diagnosed through patient history, examination and confirmation via a sleep laboratory study called polysomnography.
The severity is defined by the number of apnoea or hypopnoea events per hour (AHI) during sleep. An apnoea event is defined as a period of no upper airway flow in or out for over 10 seconds. A hypopnoea is a period of decreased airflow of at least 50% that results in a decreased arterial oxygen saturation of 4% or more due to the partial airway obstruction.
Classification of Apnoea/Hypopnoea Index (AHI)
Normal AHI: <5 events/hour
Mild AHI: 5-15/hour
Moderate AHI: 15-30/hour
Severe AHI: >30/hour
Incidence: According to the British Lung Foundation document, OSA is common, affecting an estimated 1.5 million adults in the UK and, yet, up to 85% are undiagnosed and, therefore, untreated [1].
Investigation: A history of loud snoring with an Epworth sleepiness score of greater than 16 and often a good history of cessation of breathing during sleep from sleep partner will suggest an OSA and a polysomnography is recommended to confirm the diagnosis.
Consequences of OSA: Untreated OSA is associated with significant risk of developing heart diseases, hypertension, metabolic diseases such as diabetes, mental health issues, automobile accidents, social issues and marital disharmony.
Non-surgical treatment
The treatment of OSA starts with a full general, medical and lifestyle history. Certain medications, such as benzodiazepine, can exacerbate OSA and, hence, an alternative medication may be required if it cannot be stopped. Lifestyle changes, including weight reduction in obese patients and cessation of smoking, are helpful as part of non-surgical treatment of OSA.
The gold standard treatment for OSA is continuous positive airway pressure (CPAP). The compliance is, however, poor due to various reasons, such as discomfort, dry throat, claustrophobia and noise from the machine. The mandibular advancement device is a non-surgical treatment of OSA but the compliance of using it is also poor.
Surgical treatment
Over recent decades, several surgical treatments have been tried, with varying success rates. These include pharyngeal expansion surgeries, tonsillectomy, palatal stiffening, tongue base reduction, mandibular osteotomy, nasal surgery, and tracheostomy.
Hypoglossal nerve stimulation implant: new treatment for moderate to severe OSA
A publication in January 2014 in the New England Journal of Medicine reported a significant reduction of AHI and improvement in sleep quality following hypoglossal nerve stimulation (HNS) implant in the treatment of moderate to severe obstructive sleep apnoea [2]. The hypoglossal nerve stimulation implant has received a CE mark in 2010 and was approved by FDA in 2014. Since then, the device has been implanted in over 40,000 patients between the USA and Europe.
NICE (National Institute for Health and Care Excellence) provided guidance for starting this procedure in the UK in 2017 [3]. Since then, the author has successfully implanted the first Inspire HNS implants in three patients. The author observed a significant decrease in AHI and improvement of quality of sleep at 12 months in all three cases. This is comparable to the findings in the Strollo et al. study [2].
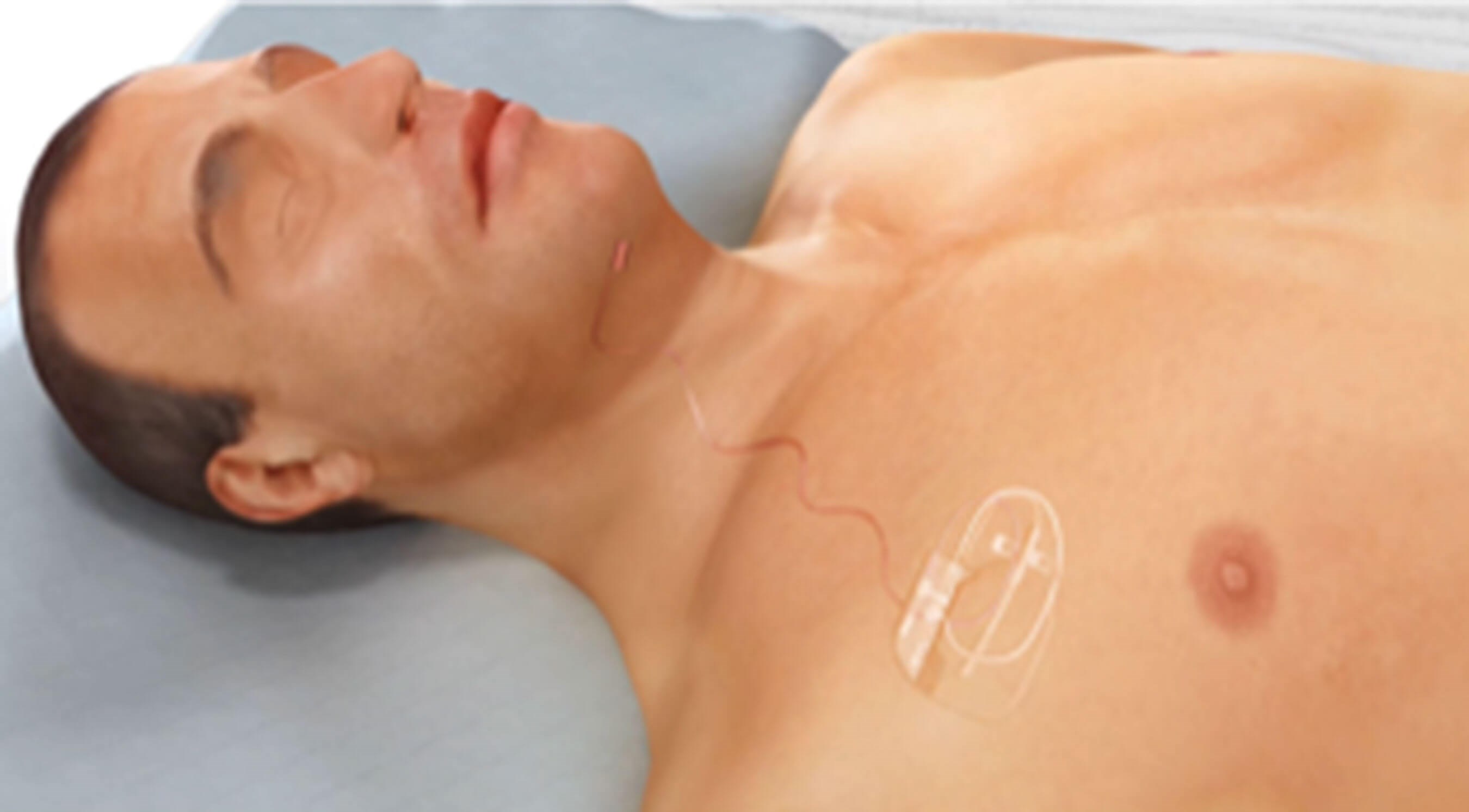
Figure 1. Diagram illustrating the chest pulse generator connected
to the neck electrode cuff by a subcutaneous cable.
What is a hypoglossal nerve stimulation implant?
This is an implantable device that has three components (Figure 1):
- An electrode cuff inserted on the branches of the hypoglossal nerve responsible for tongue protrusion (nerve supply to geniohyoid and genioglossus) through a 5cm incision on the right side of the neck, about 2cm below the inferior border of the right mandible (Figures 2a-d).
- An impulse generator placed subcutaneously over the pectoralis major muscle on the right chest wall through an incision made 5cm below the right clavicle and 3cm lateral to the lateral border of the sternum on the right side (Figures 3a-b).
- And a sensor placed between the external and internal intercostal muscles in the second intercostal on the right side (Figures 4a-b).
- The chest pulse generator is then connected to the neck electrode cuff via a cable tunnelled subcutaneously (Figure 5).
- The device is then activated four weeks postoperatively, as shown in Figure 6.
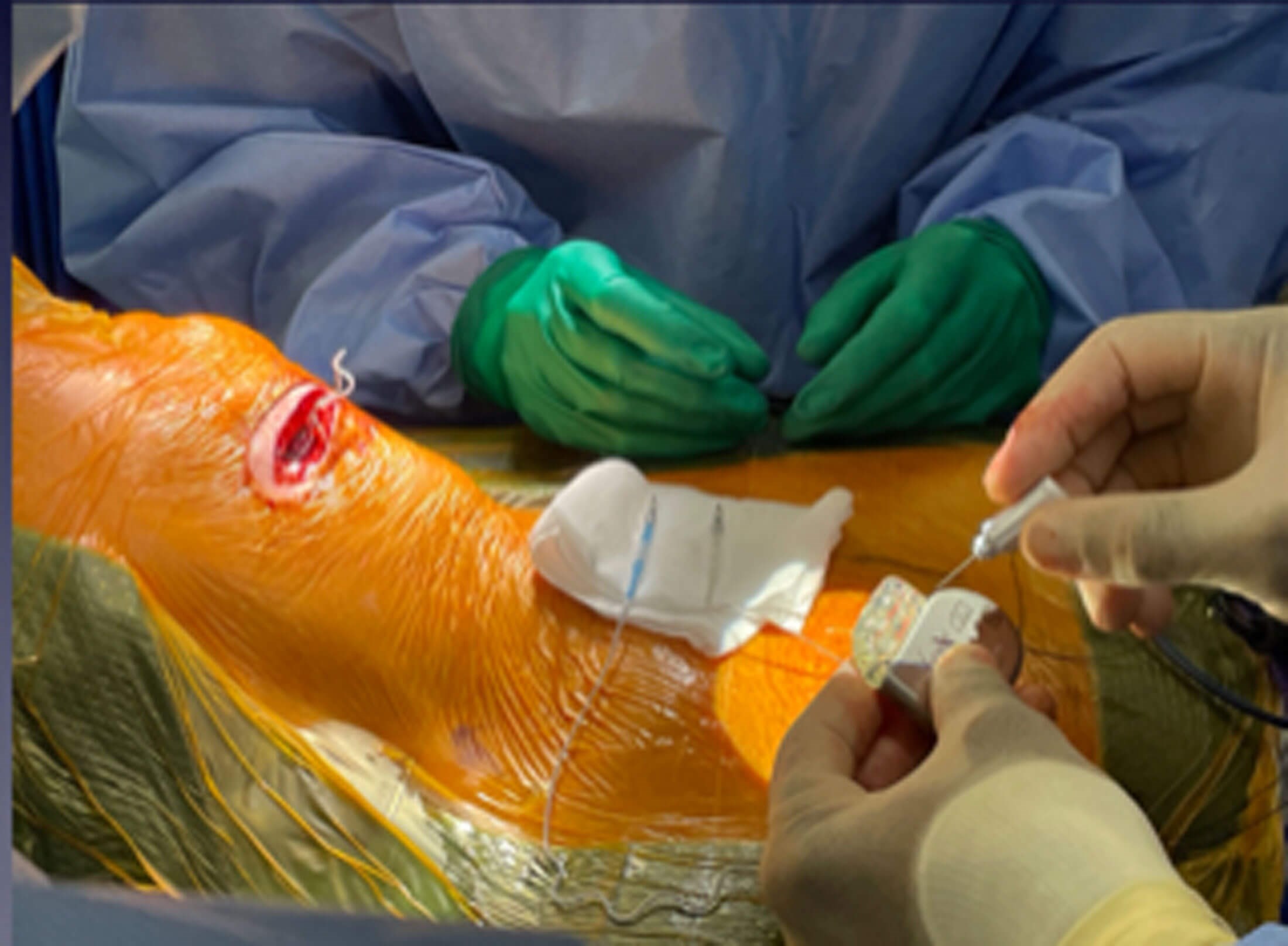
Figure 2a. Illustrating the neck incision on the right side,
about 2cm inferior to the lower border of the mandible.

Figure 2b. Illustrating the nerve supply to tongue protrusion
muscle where the electrode cuff is placed.
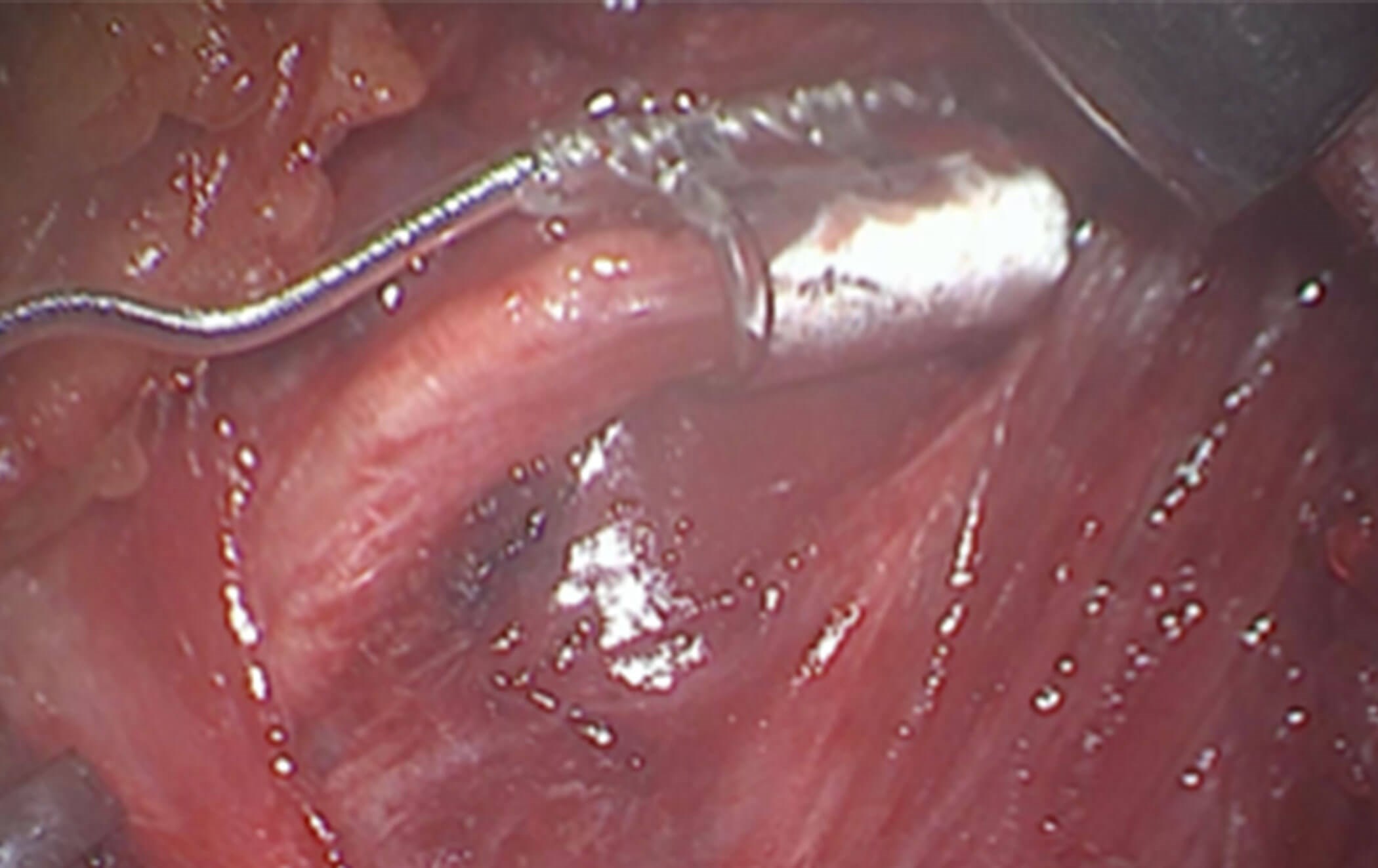
Figure 2c. The electrode cuff is placed on the nerve supply to the genioglossus,
transverse and vertical muscle of the tongue and geniohyoid muscles.
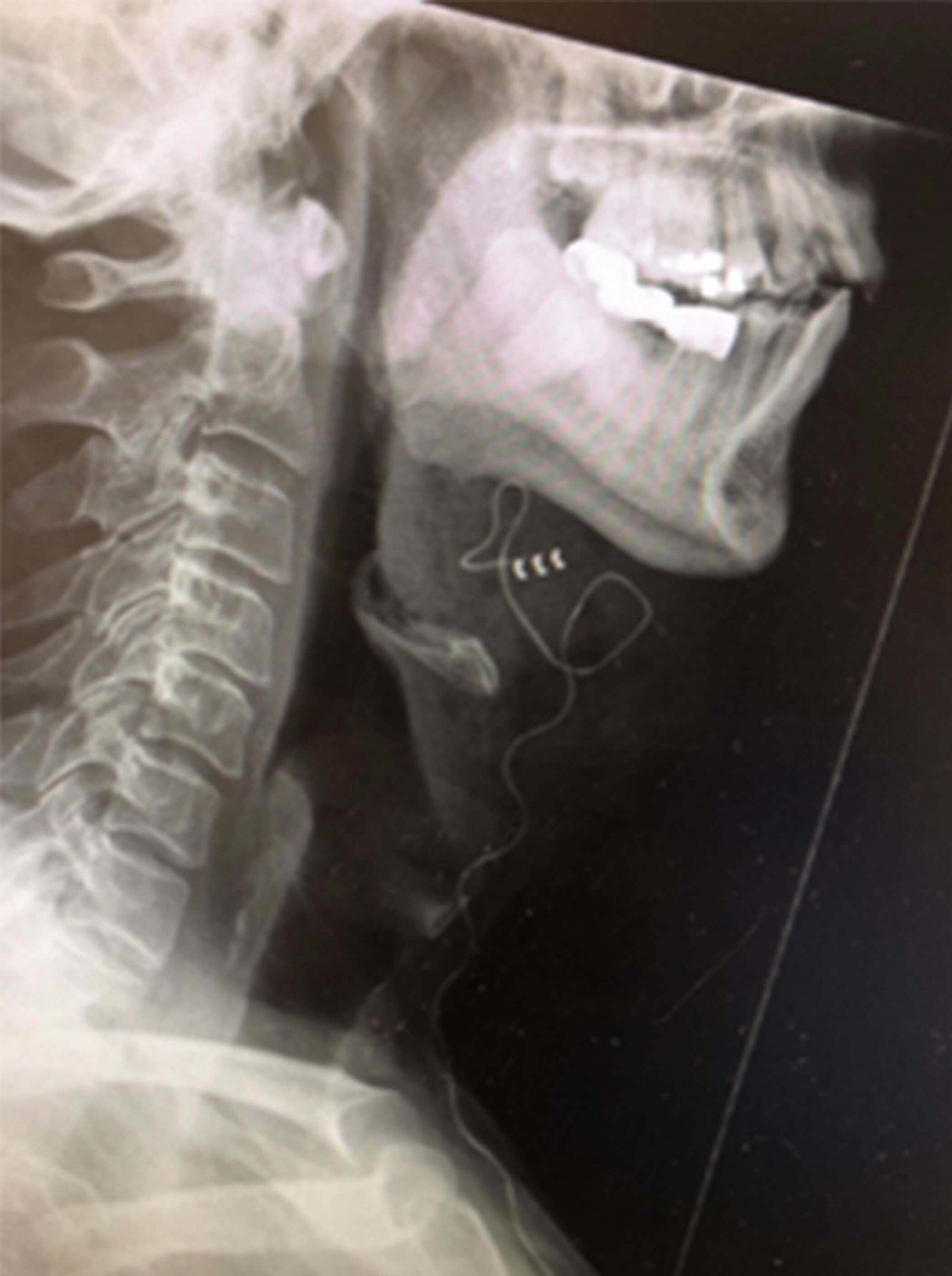
Figure 2d. Postoperative right lateral neck X-Ray showing the electrode cuff
placed on the anterior branches of the hypoglossal nerve.
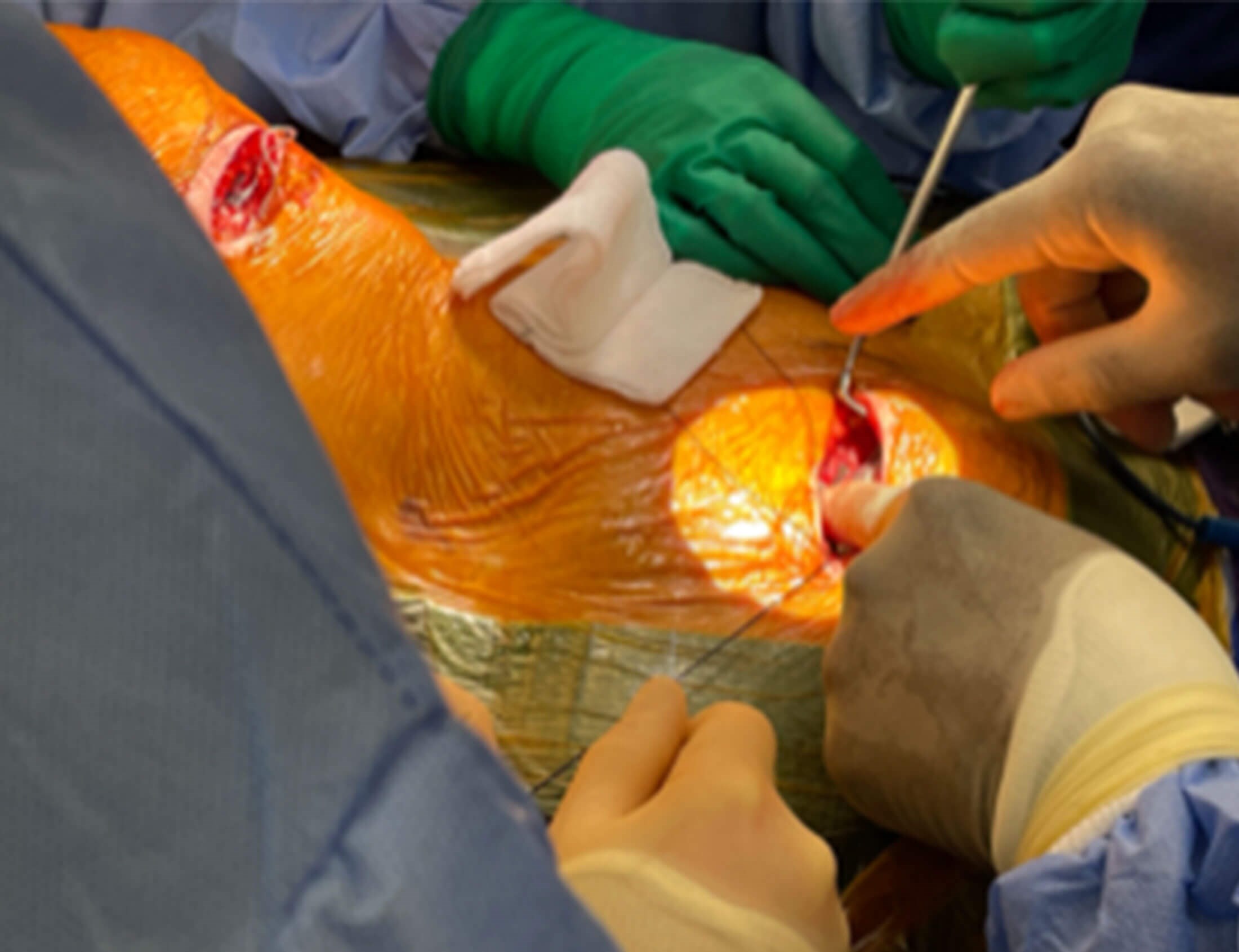
Figure 3a. Operative picture illustrates placement of the pulse generator in a
subcutaneous pocket in the right chest wall about 5cm below the clavical and
3cm lateral to the lateral border of the sternum on the right side.
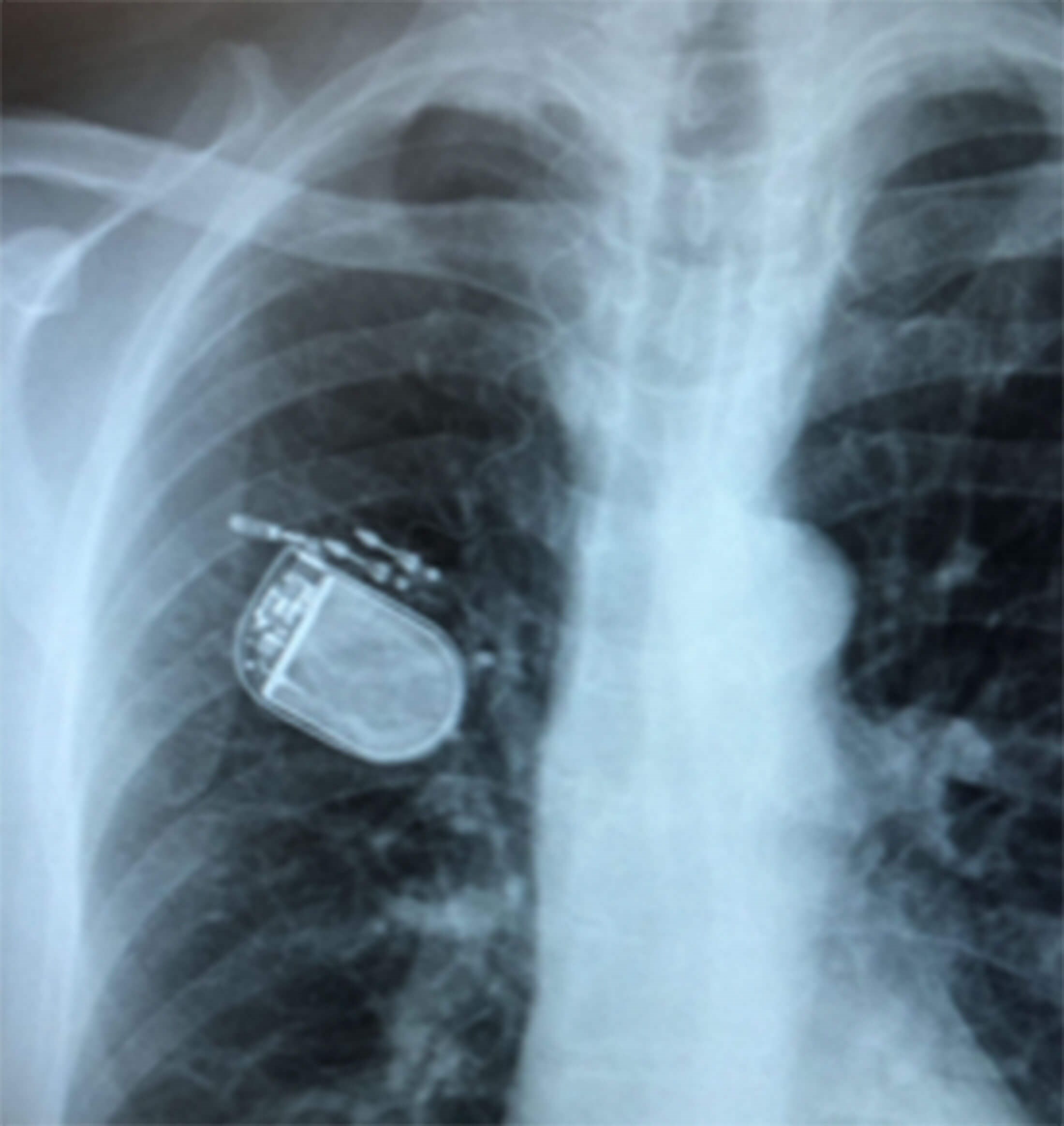
Figure 3b. Postop chest X-Ray showing the pulse generator in situ.
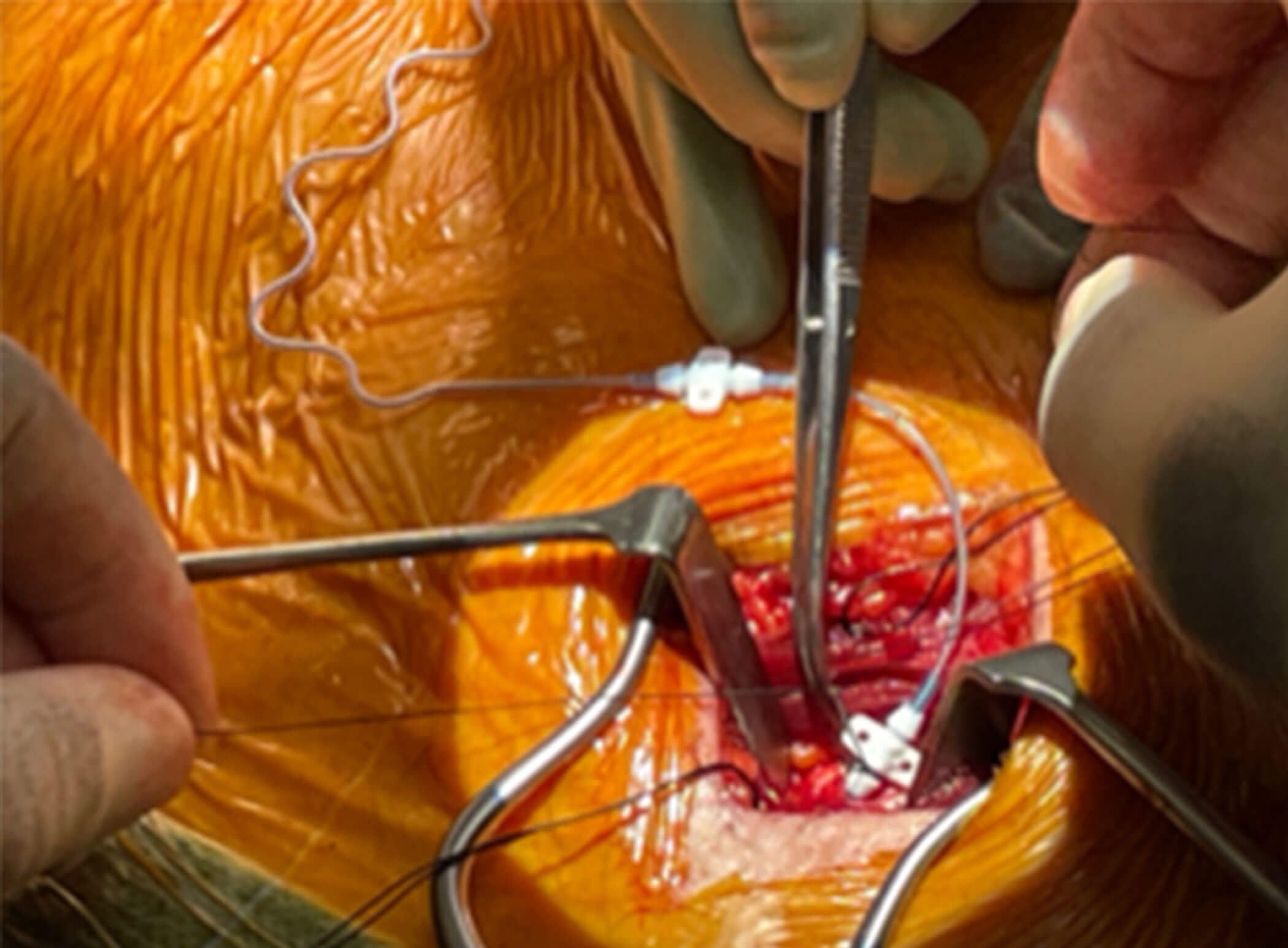
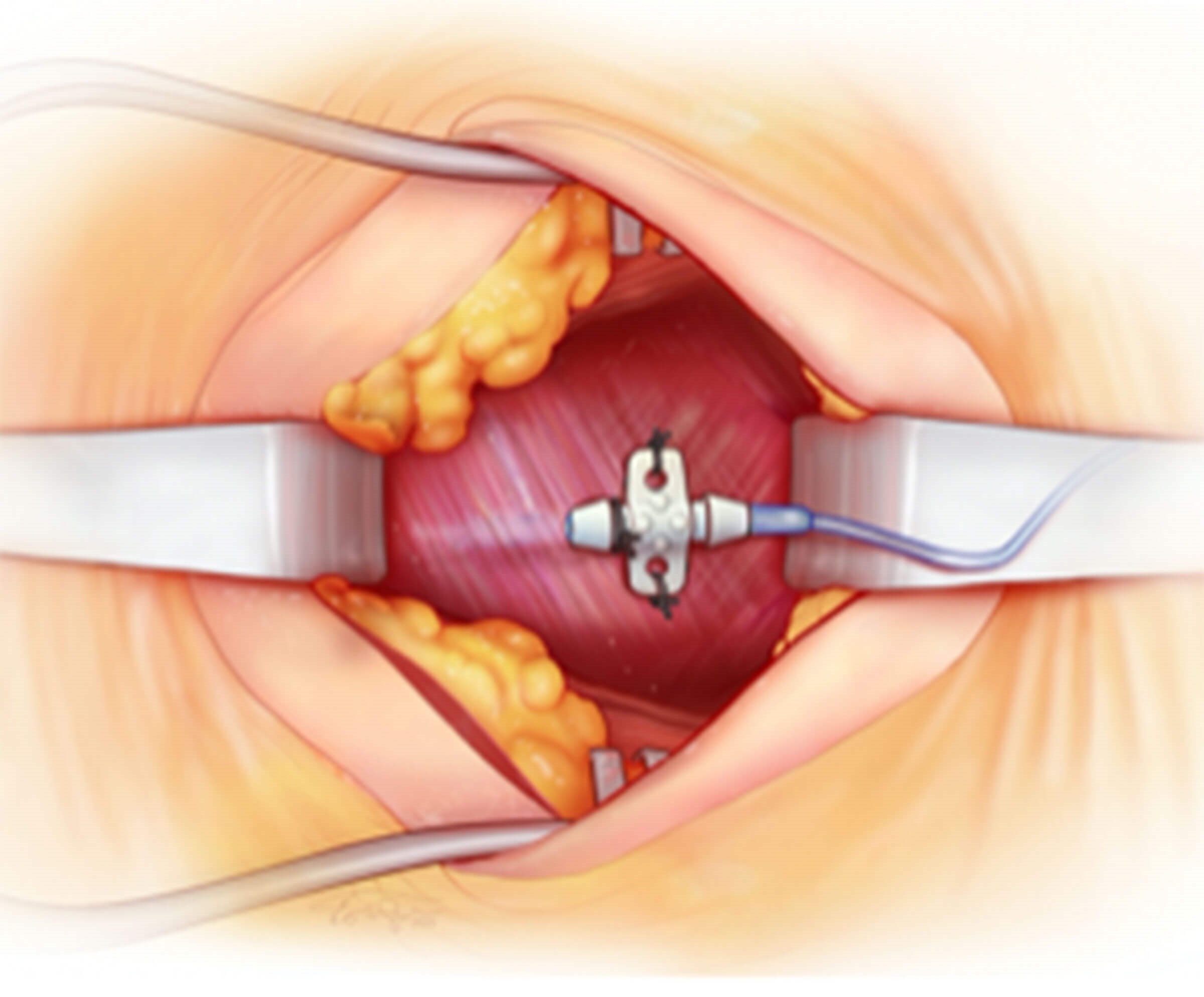
Figure 4a and 4b (top and above). Operative picture and illustration showing
insertion of the sensor electrode in the right second intercostal space
between the external and internal intercostal muscles.
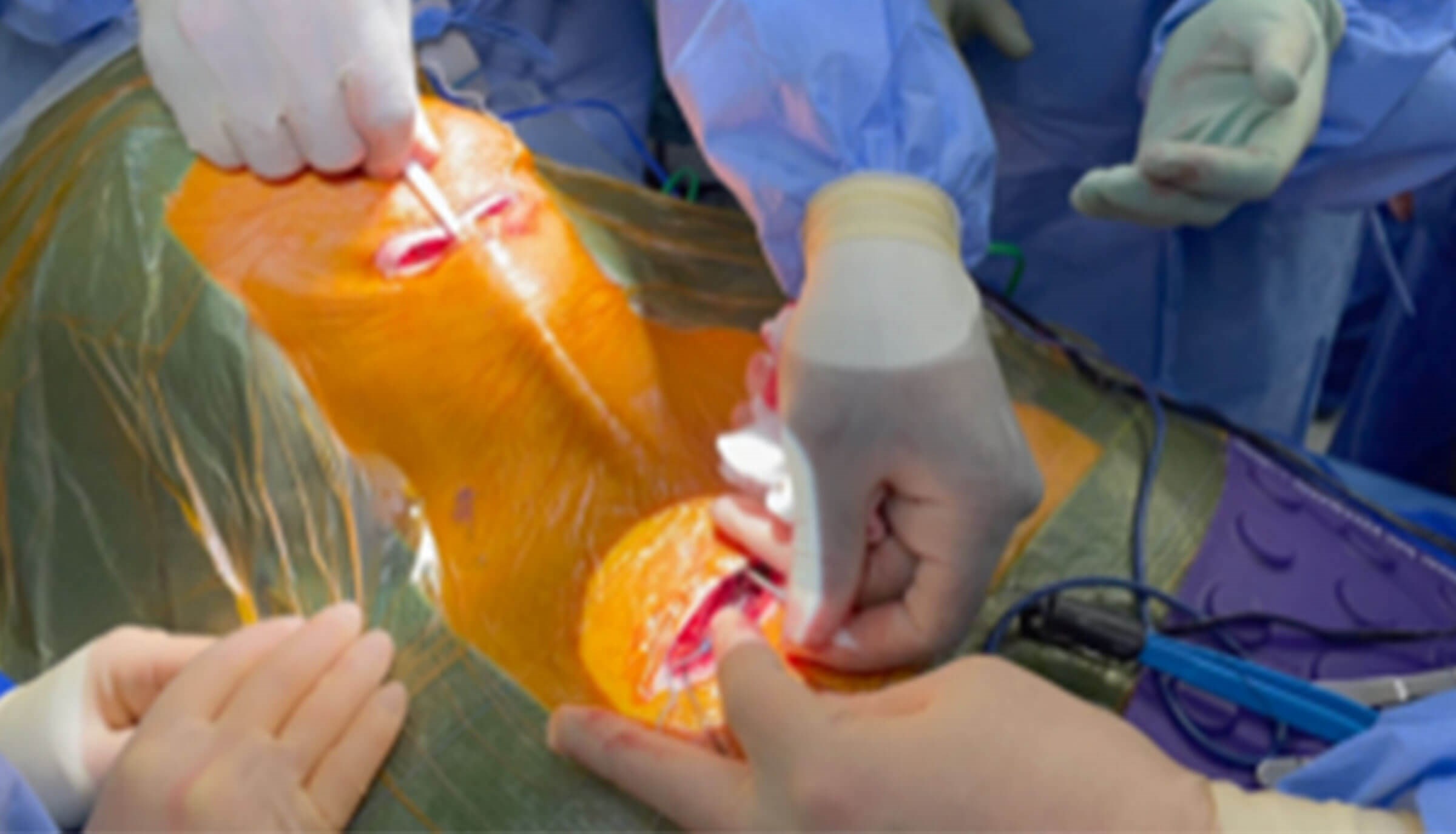
Figure 5. Intraoperative picture showing tunnelling of the electrode cable between the neck
and chest incision to the connect the pulse generator to the electrode cuff in the neck.
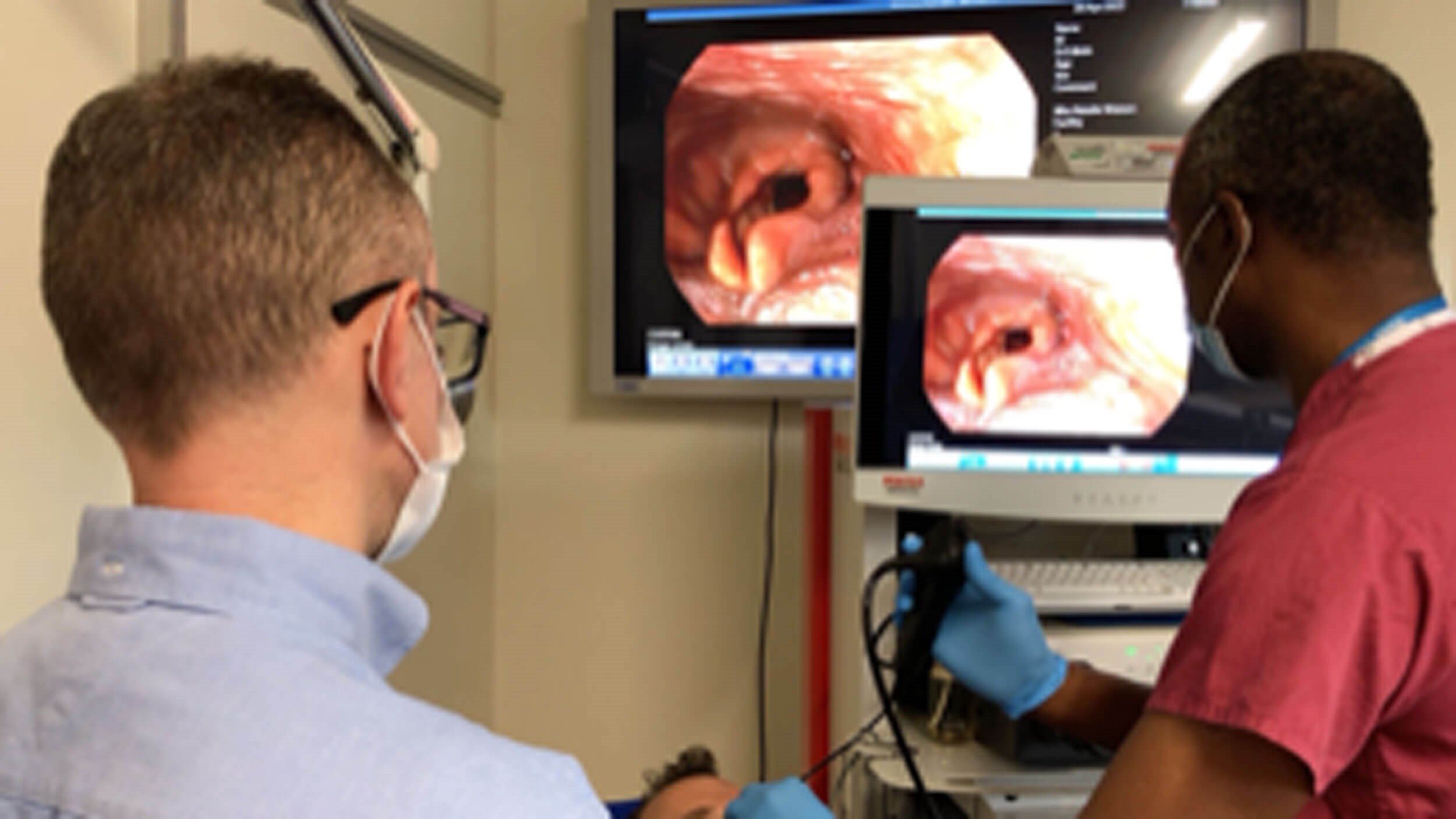
Figure 6. The hypoglossal nerve being stimulated, and tongue
base protrusion examined endoscopically post activation.
How does it work?
The implant works by stimulation of the tongue muscles responsible for protruding the tongue forward to prevent it from prolapsing into the oropharynx and causing obstruction.
What are the criteria for implanting the device?
Inclusion criteria
- Failed CPAP
- AHI 15-65/hour
- BMI less than 35
Exclusion criteria
- Central or mixed apnoea less that 25%
- Significant neurological disease
- Significant facial malformation
- Significant mental health issues to prevent understanding
- Significant chronic obstructive airway disease
- Significant large tonsils - grade 3 or 4
Complications
Injury to marginal mandibular nerve branch off the facial nerveInjury to the hypoglossal nerveWound injectionBleedingPneumothoraxDevice failureConclusionAccording to the recent research, this procedure is safe and shows a significant improvement in AHI, ODI and quality of sleep, with an outcome similar to CPAP [2]. The benefits last over five years, as shown in a study by Woodson et al. [4]. Inspire hypoglossal nerve stimulation is now an approved surgical treatment for OSA in Europe and the USA, with 40,000 patients being treated with this device. Complications are rare and often temporary: for instance, infection, haematoma or neurapraxia of the hypoglossal nerve or marginal mandibular branch of the facial nerve.
References
1. British Lung Foundation. Obstructive Sleep Apnoea (OSA). Toolkit for commissioning and planning local NHS services in the UK. 2015.
www.blf.org.uk/sites/default/files/
OSA_Toolkit_2015_BLF_0.pdf
Last accessed April 2024.
2. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Eng J Med 2014;370(2):139-49.
3. NICE. Hypoglossal nerve stimulation for moderate to severe obstructive sleep apnoea: Interventional procedures guidance [IPG598]. 2017.
www.nice.org.uk/guidance/ipg598/
informationforpublic
4. Woodson BT, Strohl KP, Soose RJ, et al. Upper Airway Stimulation for Obstructive Sleep Apoea: 5-Year Outcomes. Otolaryngol Head Neck Surg 2018;159(1):194-202.
Acknowledgements:
- Laryngology team at Guy’s and St Thomas’s Hospital
- Natalie Watson, Consultant Laryngologist
- Elfy Chevretton, Consultant Laryngologist
- Shiying Hey, Senior Laryngology Fellow
- Krizzia Lammas, Laryngology Nurse Practitioner
- Professor Joerg Steier, Sleep Physician
- Inspire Medical system training support team





