Bone anchored hearing aids are becoming increasingly more commonplace with more than 120,000 users worldwide. These devices are based on the principle of direct bone conduction, where sound is transmitted directly through the skull via a titanium implant to the inner ear. These are indicated in those with a conductive or mixed hearing loss, who may have absent, closed, inflamed or chronic discharging ears and in those with profound unilateral sensori neural hearing loss.
As bone anchored hearing programmes have grown worldwide, so too has the need for equipment which can objectively measure the output of the aid. In clinics, a bone conduction subjective listening check on a test rod or head band does not always reveal underlying technical performance mismatches. Bone conduction transducers are generally more likely than air conduction transducers to suffer from technical issues if dropped or mishandled. Clinicians may not be confident at times that the device is performing to specification. This is especially important for paediatric devices where the child may not be able to report a fault.
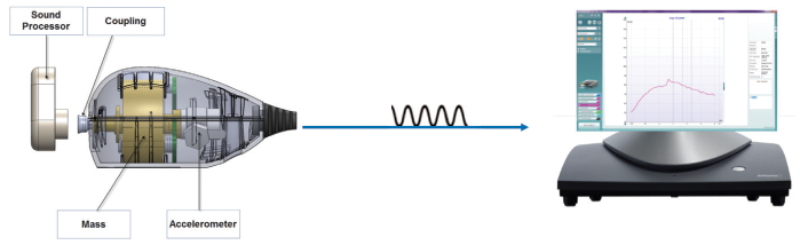
The arrival of the skull simulator allows clinicians and audiologists to be confident that the patient’s device meets specification. Historically, skull simulators were prohibitively expensive, mostly restricted to use in large labs, with most clinical programmes unable to afford this equipment. The simulator looks like an egg-shaped mass with an abutment, and is somewhat similar to the 2cc-coupler that simulates the ear-canal for standard hearing aid verification, and objectively measures the performance of the aid.
In principle the skull simulator may provide a more efficient clinic and a more satisfied patient. It can be used in preoperative evaluation to assess the technical settings of trial devices, and postoperatively to measure if the device is working as intended. Due to increased affordability this technology is increasingly found as part of bone anchored programmes.
In this spotlight we look at how the skull simulator works and the potential value to bone anchored programmes. We talk to Dennis Mistry of Interacoustics.
How does it work?
Q&A with Dennis Mistry, Clinical Product Manager, Hearing Aid Fitting, Interacoustics.
Dennis, you work for the company who have developed this product with Oticon Medical. Can you tell us a little about how it works?
The Skull Simulator simulates the mechanical impedance of the head. The Skull Simulator transduces the vibrations from the bone anchored hearing instrument into electrical signals, similar to how a 2cc-coupler microphone converts sound waves into electrical signals, to allow an objective display of its performance in the Interacoustics software.
How is this product useful in the clinical setting?
Previously we relied on subjective assessment of the devices and this can prove to be problematic when patients return with complaints and when knowing what amplification they are receiving from their processor. This product helps us to quickly acknowledge that the device is / isn’t functioning as intended. This brings confidence not only to the clinician and the patient but to the parent, teacher and anyone else involved in the patient’s wellbeing / rehabilitation / education process. This is because they know that the patient is receiving suitable amplification to allow optimal performance.
There can also be a long wait time for device repair which is subjectively assessed to be faulty and this can lead to a depleted loan stock – thus affecting the service which your patients are receiving. Through use of the Skull Simulator we can quickly check and know if the device is working or not and if necessary send it complete with a report to the manufacturer for repair. This helps to triage the amount of devices sent for service thus saving money for the clinic.
What are the main operating differences for a clinician when testing via a skull simulator compared to a 2cc-coupler that simulates the ear canal?
As the Bone Anchored device is outputting in vibration we are looking at our measure in Newton force, specifically dB µN. This is completely different from the dB SPL or dB Gain we traditionally see in our hearing aid technical measures.
We are working with Bone Anchored Hearing Solution manufacturers and have developed specification sheets similar to how you would verify hearing aid function. This cooperation has even increased towards software integration where Oticon Medical’s software, Genie, can now quickly launch the Interacoustics Affinity Suite to perform technical measurements with the Skull Simulator.
Images: A top down systematic view of the configuration to use the Skull Simulator.
But traditionally it is recommended that the Output Force Level 90dB (OFL90) is measured – similar to an OSPL90, Full-on-gain 50dB (FOG50), Total Harmonic Distortion and Processing Delay. It is also good to measure the end settings following fine tuning at 65dB.
These measures can help to determine whether the device is working correctly according to the manufacturer’s specification. They can also enable longitudinal monitoring and accountability of the final amplifications settings prescribed – this is crucial as there is no fitting target or standardised process for fitting Bone Anchored Hearing Solutions.
How long does it take to run the tests?
The test procedure is very quick, the device works within the Interacoustics HIT module and is run via a fast automated process which takes no longer than 40 seconds once the device is coupler to the simulator and placed in the Test Box. Normative ranges can also be enabled so that a benchmark / baseline of device performance, when set to factory settings, can be logged. This can allow for non-audiologists or non-technicians to run this measurement following a trial / loan period to determine whether the device should be returned back to the loan stock or sent for repair.
Thank you to Dennis Mistry for taking the time to contribute to this spotlight.
This product offers a new solution to measuring the performance of a bone anchored hearing device. We welcome this and further innovation, and would be particularly interested to see an affordable, portable skull simulation test system which may ultimately allow patients, their parents or teachers of the deaf to become more involved in the monitoring of device performance. We look forward to hearing more.
‘Spotlight on Innovation’ is an informative section to provide insight and discussion on recent advances in technology and research and does not imply endorsement by ENT and Audiology News.