Will this pill cure tinnitus? Bonnie Millar describes one trial that has investigated the possibility whilst describing the path of drug trials in the UK.
Background
In the last quarter of 2014, a clinical trial (QUIET-1; ClinicalTrials.gov Identifier: NCT02315508) commenced recruitment. The aim of the trial was to test the effectiveness of a capsule (containing the Autifony Therapeutics Ltd AUT00063 compound) taken orally to treat chronic subjective tinnitus. This multicentre trial was designed to recruit up to 152 participants across 18 NHS sites, including the Nottingham University Hospitals NHS Trust (NUH).
The logistics of coordinating this trial was demanding: there were many assessments, multifarious inclusion criteria, and a large study team spanning numerous NHS departments within each site, as well as several external organisations. Furthermore, as previous reports [1,2] and studies [3] have highlighted, good clinical trial methodology which has the flexibility to adapt to the different project stages, was paramount to the coordination of site activities. This article reflects on how the trial was managed locally at NUH to resolve these logistical challenges.
Communication
From the outset, a single point of contact proved invaluable to maintain clear communication channels for staff and participants. The study coordinator was this single clear point of contact, always accessible, and with oversight of every participant activity from pre-screening through to the last follow-up visit.
“From the outset, a single point of contact proved invaluable to maintain clear communication channels for staff and participants.”
This central contact, combined with clear study team role definition (each task owned), avoided duplication of effort and tasks not being actioned in a timely manner. A further practical measure was the use of a participant chaperone to accompany participants on the longer complex visits (day 1 and day 28) with their busy timetables and multiple assessments in several locations; by supporting and assisting the participant in this manner, their commitment and interest in the study was hopefully also fostered.
Trial logistics
No less than 25 people from seven departments were on the site delegation log (including two investigators from ENT, audiologists, nurses and pharmacists). The Nottingham site was the only one to perform phEEG and ABR tests (Figure 1).
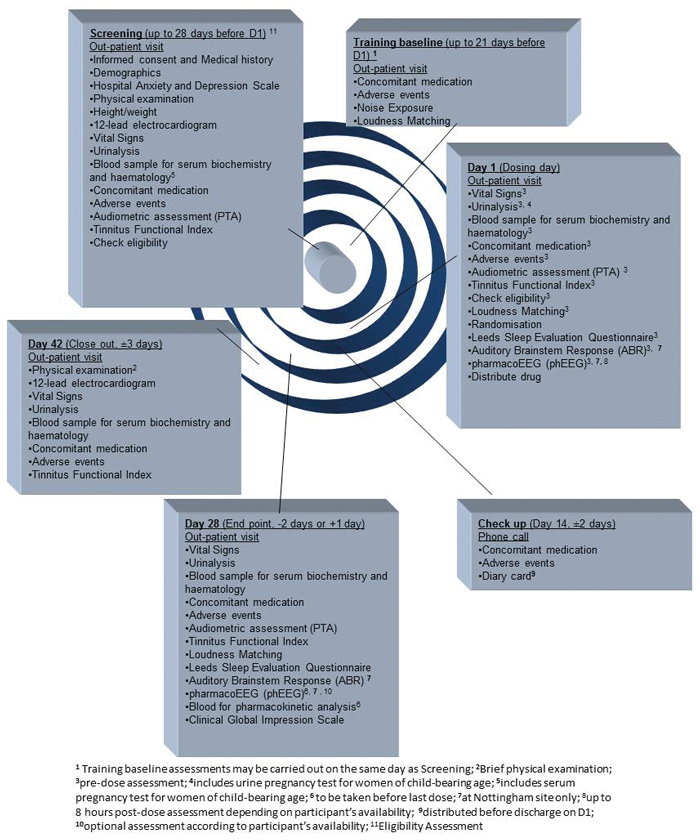
Figure 1. Table listing all the assessments and visits for the participants.
The requirements of hospital clinical pathology, four clinical research organisations (CROs), the sponsor (Autifony Therapeutics), and an external pharmaceutical services company, also had to be considered. Each CRO had a specific data collection / reporting remit, with Cromsource having oversight of the trial master file and trial data. For each visit, a clearly itemised itinerary with every task timed, was produced to ensure obstacles to participant engagement and staff involvement were reduced.
Existing hospital systems often proved impractical for study purposes, necessitating the development of alternative processes; for example, for referrals and collection of results.
Consequently, it proved possible, for instance, to accommodate one day 1 visit (consisting of seven appointments with clinicians from five departments over 11 hours), one day 28 visit (consisting of five appointments with clinicians from five departments over four hours) and two eligibility assessment appointments (with clinicians from three departments over three hours) on the same day through tight scheduling and a participant chaperone. All recruited participants remained fully engaged and committed to the study.
Recruitment
Recruitment into clinical trials is widely recognised as an area which requires considerable design, planning and resources to implement [4]. For QUIET-1 recruitment, pre-screening and enrolment activities ran concurrently.
During the recruitment phase, the number of queries, telephone interviews, volunteers in receipt of the participant information sheet (PIS), those in receipt of eligibility assessment appointments, and recruited participants, were monitored and cross-referenced. If recruitment appeared to be stagnating, new advertising initiatives were instigated. The effectiveness of the strategies used was evaluated by the number of queries, eligibility assessment appointments and recruited participants yielded, and this data informed which tactics were later reused or discontinued (Figure 2).
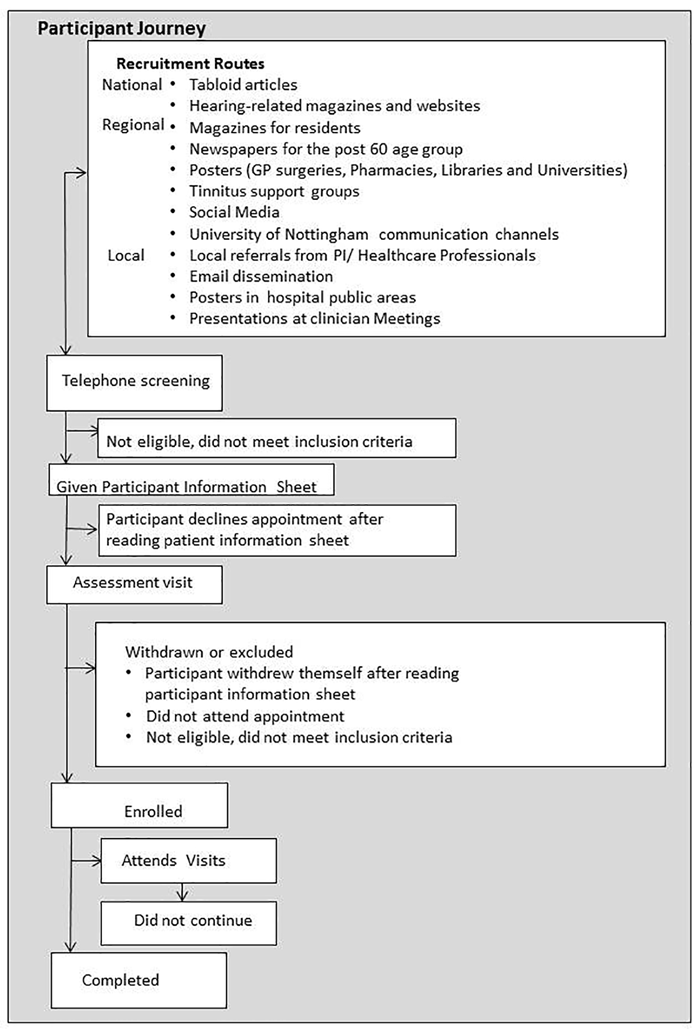
Figure 2. This chart details the participant journey.
“Recruiting subjects with sub-chronic tinnitus was very challenging. It was really important to capitalise on events like the annual Tinnitus Awareness Week, but then maintain the momentum across all recruiting sites.” (Alice Grant, Senior Clinical Project Manager, Autifony Therapeutics Limited)
Data collection
“I would say that it is definitely important to keep site files well organised from the very beginning of the study. This makes the life of the clinical research associate (CRA) much easier. It also means that if there are any issues with the files (wrong templates used, documents missing etc.), a neat site file makes these problems much easier to detect and also easier to resolve.” (Gill Smith, CRA, Cromsource)
“Having trial-specific participant folders organised per visit, containing the standardised specially developed source data sheets, forms, questionnaires and participant information leaflets mitigated the potential for missing or inaccurate data.”
Having trial-specific participant folders organised per visit, containing the standardised specially developed source data sheets, forms, questionnaires and participant information leaflets (everything in its place) mitigated the potential for missing or inaccurate data. Furthermore, it limited the amount of participant time required for appointments, and proved a lighter touch for staff involvement. Upon reviewing processes after three months, study-specific labels with the clinical details for the required samples were developed to further save time and ensure accuracy. This, combined with internal monitoring and prompt query chasing, ensured clean data. The shorter the interval between data capture, input, checking and correction, the greater the success rate in query resolution.
Accordingly, external reporting by the CRO highlighted no discrepancies between the telephone randomisation system and the electronic case report form (eCRF) with regard to audiology markers, and the reconciliation of both was quick and straightforward. Likewise, external medical reviews were accomplished with minimal fuss and incidental actions. Clinical research associates observed during monitoring visits, that the Nottingham files were the neatest that they had seen!
References
1. Farrell B, Kenyon S, and Shakur H. Managing clinical trials. Trials 2010;11:78.
2. Pocock SJ. Clinical Trials: A Practical Approach. Chichester, UK; John Wiley & Sons; 1983.
3. Campbell MK, Snowdon C, Francis D, et al (the STEPS group). Recruitment to randomisedtrials: strategies for trial enrolment and participation study. The STEPS study. Health Technology Assessment 2007;11(48).
4. McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7:9.
Declaration of Competing Interests: None declared.
ABOUT THE AUTHOR
Bonnie Millar holds degrees from Trinity College, Dublin and the University of Nottingham. She joined the NIHR Nottingham Hearing Biomedical Research Unit in 2014 to work on the QUIET-1 clinical trial, coordinating recruitment and trial activities for the Nottingham site. In her current role, Bonnie is applying her expertise in research methodology and textual analysis, to facilitate the transfer of research data pertaining to studies conducted within the unit’s tinnitus research area into a single data repository.
SUMMARY
-
Clear communication channels are vital.
-
Logistics should be thoroughly planned to maximise clinicians’ time.
-
Using trial-specific systems will reduce the impact on participant and staff time and facilitate continued engagement with a study.
-
Monitoring and vigilant data collecting are essential to the orderly delivery of the study aims.




