This article discusses requirements for an early congenital cytomegalovirus (cCMV) detection pathway to ensure children do not miss out on the opportunity for timely diagnosis and treatment. The pathways described are currently in use in England and apply to well babies (i.e. babies who were not admitted to neonatal intensive care units) not already diagnosed with cCMV.
Congenital cytomegalovirus (cCMV) is the most common non-genetic cause of sensorineural hearing loss (SNHL) in children and currently the only cause for which there is a medical treatment available to prevent further hearing loss. Randomised controlled trials showed that antiviral treatment needs to be started within the first four weeks of life [1]. Recent evidence may support a three-month cut-off [2]. A modest benefit of the antiviral medication to neurodevelopment was also reported.
CMV screening tests are mandatory in newborns with hearing loss in some states of the USA, to avoid missing the treatment window. Missing cCMV has become an area for litigation. The NICE-approved aetiological investigation guidelines written by the British Association of Audiovestibular Physicians include timely investigations for cCMV (see www.baap.org.uk). The British Academy of Audiology Quality Standards in Paediatric Audiology states services must have clearly defined pathways and protocols for early cCMV diagnosis (see www.baaudiology.org). Early detection and management of major causes of hearing loss are also a WHO priority (see World Report on Hearing www.who.int/publications/i/item/9789240020481).
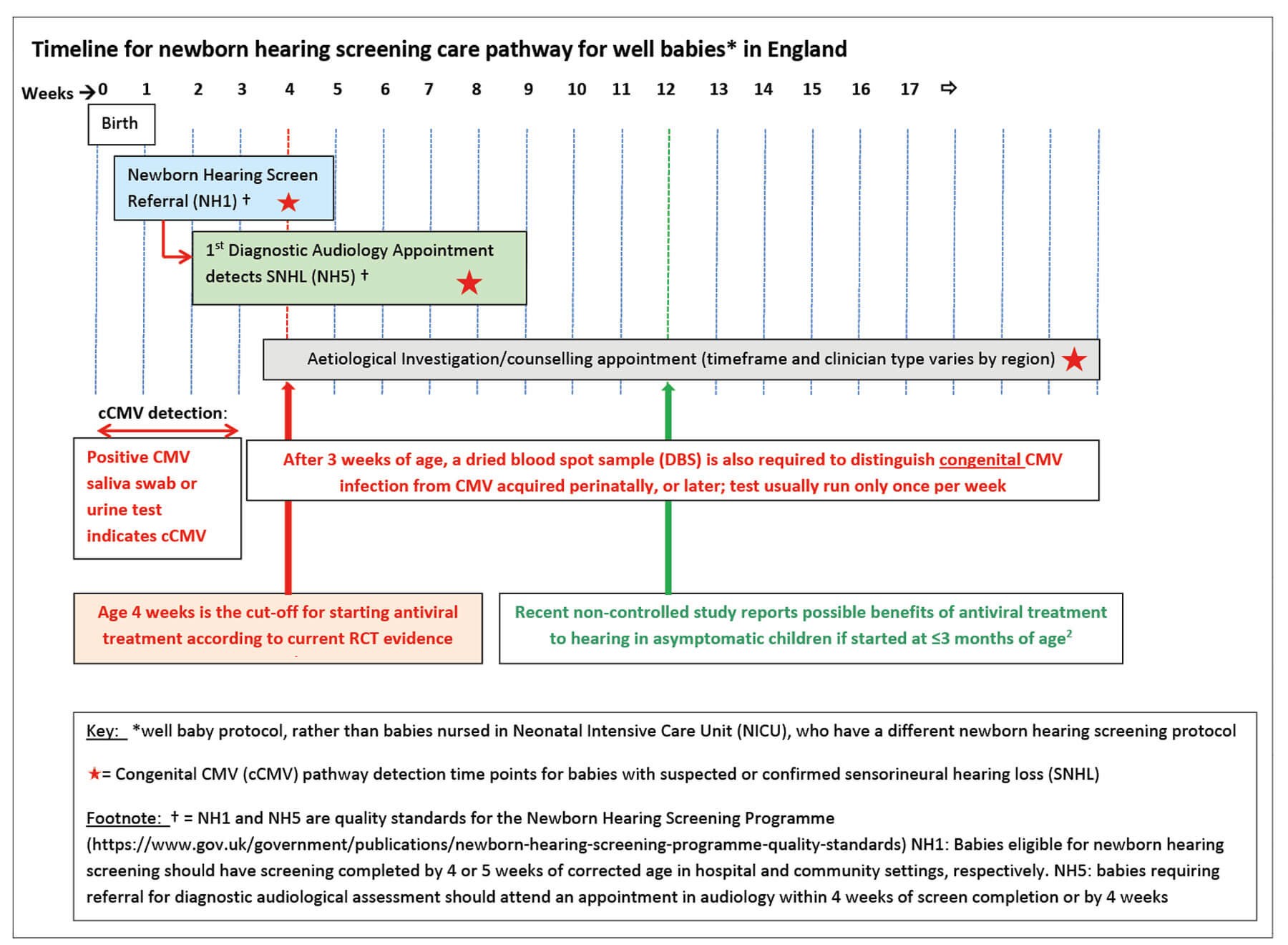
Figure 1.
The challenge
In the UK, the newborn hearing screen is completed shortly after birth, and by age four weeks in the hospital setting or five weeks in the community setting (Figure 1). If the hearing screen, audiological assessment and CMV tests are done early enough, treatment can be initiated in time, provided the process is efficient and rapid.
In December 2022, a survey was circulated to paediatric audiology departments and clinicians investigating the aetiology of SNHL in England to explore practice in this field. Responses were received from just over half of departments in England. Almost half of audiology centres did not have an early detection pathway for babies referred from the newborn hearing screen (personal communication, Heather Bailey). Barriers to having a pathway vary from lack of access to saliva swab tests to lack of trained professionals to offer tests and action results. Inequality in access to cCMV detection and treatment can be addressed by sharing expertise, and a multidisciplinary quality improvement project. Various models have been established, and the sample used and the professional taking the sample differ according to resources available locally. If you work in an area without access to an early detection pathway and wish to develop one, the necessary requirements are listed in Figure 2.
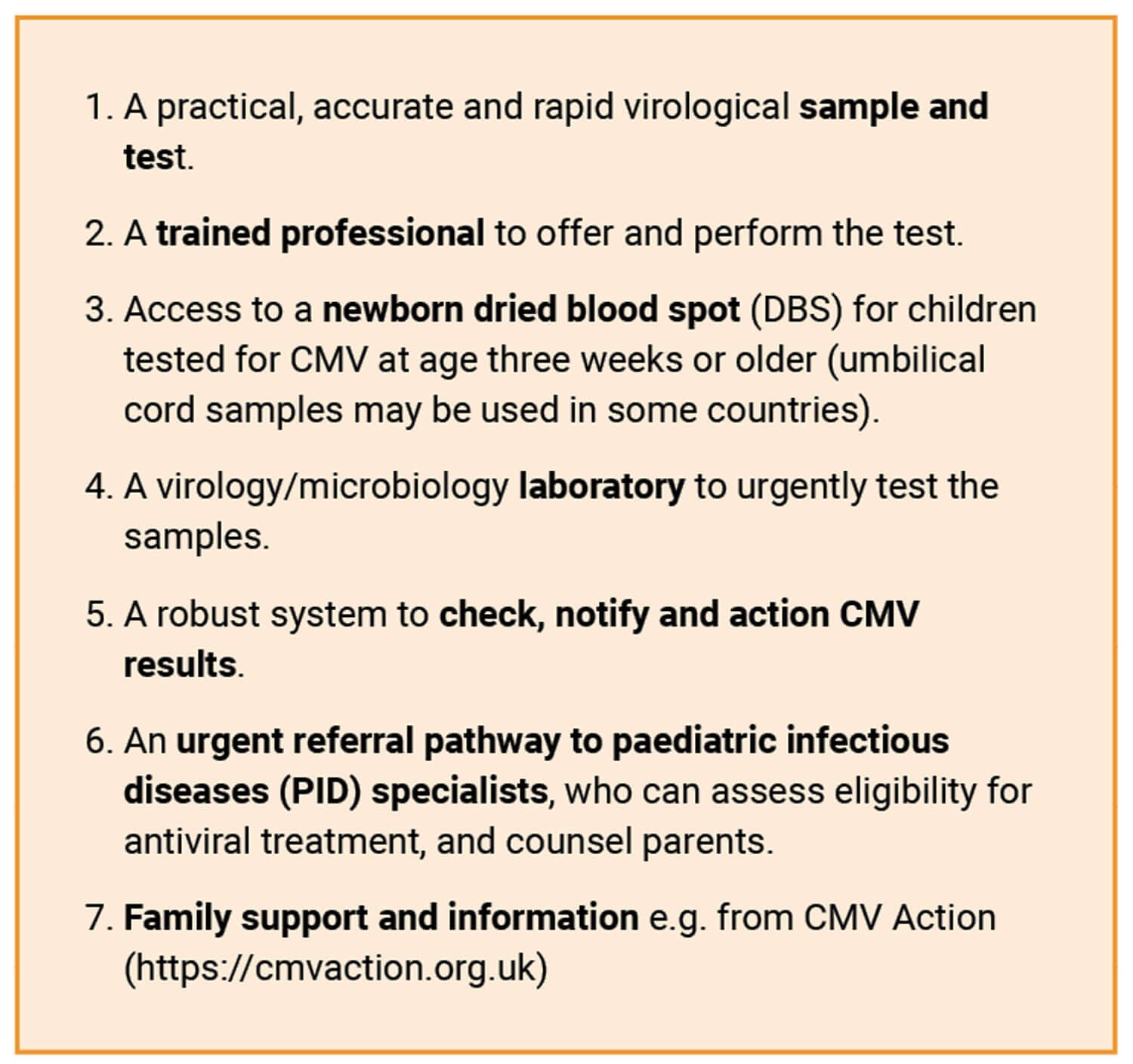
Figure 2: Requirements of an early cCMV detection pathway.
The CMV sample and test
cCMV is diagnosed when a sample taken within the first three weeks of life is positive for CMV (e.g. polymerase chain reaction (PCR) for CMV DNA). A positive CMV sample taken after age three weeks could reflect congenital, or peri- or postnatally acquired CMV infection (which is not a cause of congenital SNHL). CMV is excreted in body fluids. Both urine and saliva samples are more sensitive than blood samples, and are also less invasive to collect. Saliva swabs are easier and quicker to obtain, and more acceptable to parents, than urine samples [3]. They can be carried out by any trained health professional including the newborn hearing screener. For a breastfed baby the swab should be taken least one hour after a breastfeed, to avoid a false positive test due to CMV in breastmilk. In the recent audit, more departments were using saliva than urine samples.
Babies older than three weeks with a positive CMV test will also need their newborn dried blood spot (DBS – which is taken in the first week of life – retrieved and tested for CMV DNA to confirm congenital infection, and parental consent for this can be taken at the time of taking the saliva sample to save time (the DBS test currently only runs weekly, increasing the time to diagnosis).
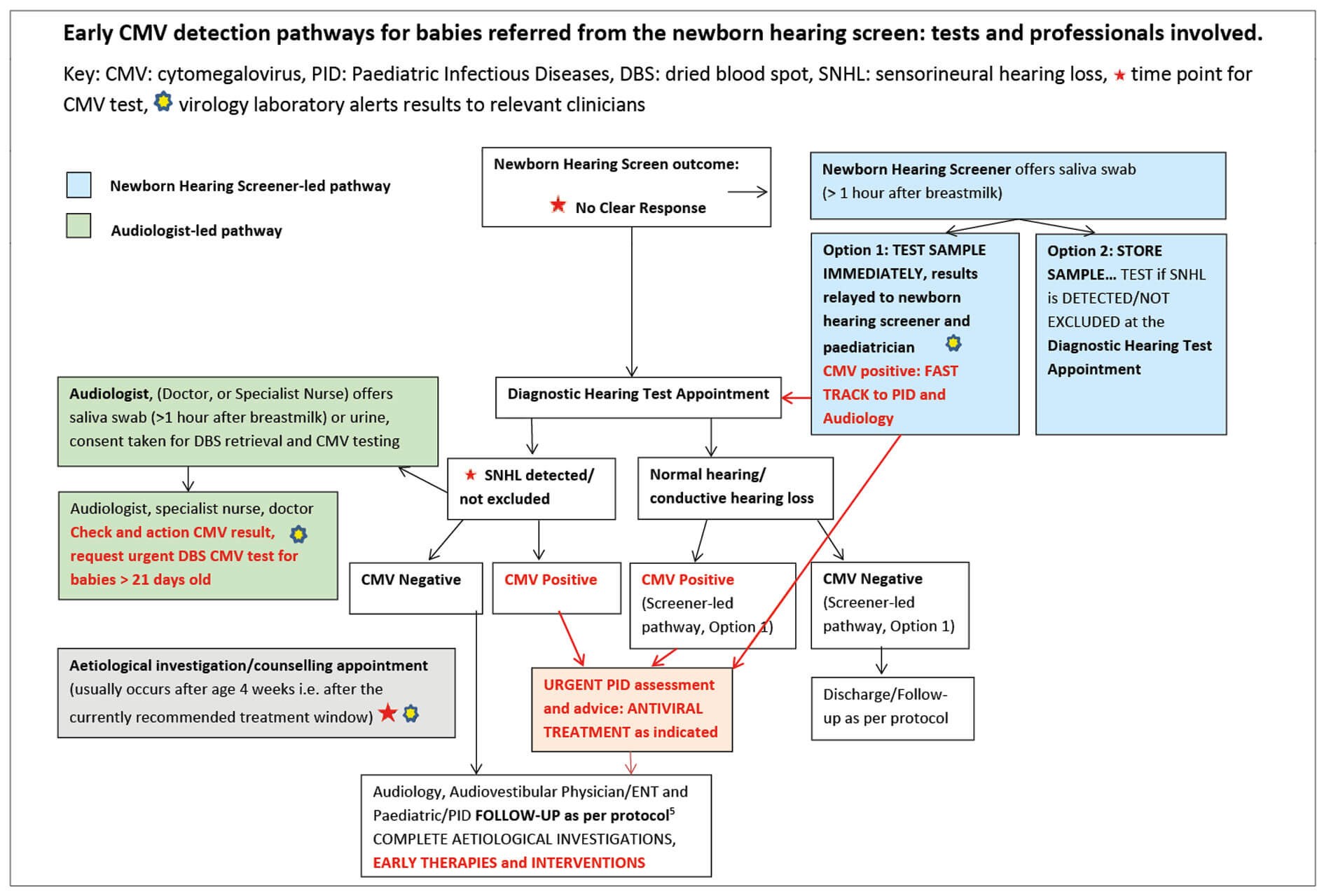
Figure 3.
The professional offering the CMV test
In the current pathways in England, tests for congenital CMV were offered to parents at three different time points and by various professionals (see Figure 3):
- At point of referral from the newborn hearing screen by the newborn hearing screener;
- When SNHL is detected, or not excluded, at the first diagnostic audiological assessment, usually by a paediatric audiologist;
- At an appointment for aetiological investigations, by a clinician trained in aetiological investigations e.g. audiovestibular physician, paediatrician, ENT doctor, specialist audiologist.
Education and training materials for the professionals involved have been developed with input from CMV Action during a pilot study, prior to adaptation for local use in clinical practice [4], (see www.nnuh.nhs.uk/publication/cmv-presentation-1-9-07-11-23). When the saliva swab is taken by the newborn hearing screener, the swab may be tested immediately, (see www.eoeneonatalpccsicnetwork.nhs.uk/wp-content/uploads/2022/02/cCMV-EoE-guideline.pdf), or stored and only tested if SNHL is confirmed.
Interpreting and actioning the result
It is essential to process the test, check and action the result urgently in view of the tight deadline for starting treatment. This requires use of confidential email +/- telephone calls and a process for virology/microbiology colleagues to alert relevant clinicians to positive results immediately.
CMV-positive results
If any sample (urine, swab, DBS) taken before three weeks of age returns a positive result, indicating cCMV, parents should be informed and an urgent referral made to paediatric infectious diseases (PID).
For infants with CMV-positive samples taken after age three weeks, the DBS CMV test should be requested urgently. It is best to also refer to PID whilst waiting for the result of the DBS, to save time. Maternal antibody tests on blood samples taken in the first trimester may also be helpful.
A positive urine or saliva result, with a negative DBS result, usually indicates a case of perinatal CMV, which does not require treatment in well babies. However, the case should still be discussed with PID colleagues because it is possible to have cCMV with a negative DBS test, owing to lower sensitivity than urine and saliva.
Infants with confirmed cCMV should be fast-tracked for detailed audiological assessment. Investigations for other effects of cCMV (brain magnetic resonance imaging (MRI), ophthalmology examination, blood count and liver function tests) should be completed urgently [5]. MRI scan requests should include both the internal auditory meati and brain.
Following assessment by PID colleagues, infants with SNHL diagnosed early with cCMV may be offered antiviral treatment, to reduce risk of further hearing loss and neurodevelopmental concerns. Regardless of decisions around antiviral treatment, all can benefit from enhanced audiological and neurodevelopmental follow-up and early interventions.
Infants diagnosed with cCMV found to have normal hearing are referred for PID assessment and advice, and audiological follow-up until age six years (minimum) [5]. Information, counselling and support should be tailored to the severity of the cCMV effects and hearing loss.
CMV-negative results
As CMV-related SNHL is often progressive in nature and may be associated with vestibular hypofunction and neurodevelopmental difficulties, negative early CMV tests are reassuring. They also enable investigations for other underlying conditions that may need treatment and genetic counselling, such as neurometabolic disorders, to be considered earlier.
An MDT quality improvement project
Early cCMV detection pathways rely on teamwork and collaboration between newborn hearing screeners, audiologists, audiovestibular physicians, ENT surgeons, paediatricians, including PID specialists, microbiologists, virologists and newborn bloodspot laboratory staff. If you don’t have a pathway in your area, it is hoped that this article aids discussions with your colleagues and funding bodies, and supports implementing one, in order to provide the best opportunities and care to infants with hearing loss, avoid delayed diagnosis and/or treatment, and reduce inequalities due to regional variations in practice.
References
1. Kimberlin DW, Chin-Yu Lin, Pablo J Sánchez, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003;143(1):16–25.
2. Chung PK SF, Soede W, Kroes A, et al. The Concert Study: Treatment of infants with congenital cytomegalovirus infection and isolated hearing loss, detected through newborn hearing screening - PD086/#1653. European Society For Paediatric Infectious Diseases 2021; Geneva.
3. Williams E, Kadambari S, Berrington JE, et al. Feasibility and acceptability of targeted screening for congenital CMV-related hearing loss. Arch Dis Child Fetal Neonatal Ed 2014;99(3):F230-6.
4. Kadambari S, Luck S, Davis A, et al. Evaluating the feasibility of integrating salivary testing for congenital CMV into the Newborn Hearing Screening Programme in the UK. Eur J Pediatr 2015;174(8):1117–21.
5. Pesch MH, Kuboushek K, McKee MM, et al. Congenital cytomegalovirus infection. BMJ 2021;373:n1212.
Acknowledgements
Vishnuga Raveendran, PhD student, UCL Great Ormond Street Institute of Child Health.
Heather Bailey, Associate Professor in Infectious Disease Epidemiology, UCL Institute for Global Health.
Catherine Magee, Clinical Project Lead – NHS Newborn Hearing Screening Programme, NHS England.
Hannah Garnett, National Programme Manager – NHS Newborn Hearing Screening Programme, NHS England.
Hermione Lyall, Consultant Paediatrician, Infectious Diseases; Professor of Practice, Imperial College Healthcare NHS Trust.
Tamsin Brown, Consultant Community Paediatrician, Cambridgeshire Community Services NHS Trust.
John Fitzgerald, Consultant Clinical Scientist, Norfolk & Norwich University Hospitals.
Shankar Rangan, Consultant & Head of Department of Paediatric Audiovestibular Medicine, Wirral University Teaching Hospital NHS Foundation Trust.
Helen Payne, Clinical Lecturer in Paediatric Infectious Diseases at Imperial Healthcare NHS Trust.
Declaration of competing interests: SW attended the RCPCH conference in 2023 as a speaker on the topic of congenital cytomegalovirus (CMV) and its association with hearing loss and her travel costs were reimbursed.