Surely the only outcome needed for post-surgical ears is a better PTA? Marcus Neudert argues there should be more to it than that.
To draw a comprehensive picture of the disease-associated restrictions in patients with chronic otitis media, audiometric outcome measures alone are not sufficient. Disease-specific health-related quality of life (HRQOL) measurements using validated questionnaires are appropriate tools to round up the picture.
“HRQOL instruments fill the gap between the individual’s subjective perceptions of disease, associated quality of life restriction and other diagnostic findings.”
Success and result measures in middle ear surgery are often based on audiometric findings only. As a result, the air-bone gap (ABG, difference of air conduction and bone conduction threshold) in pure tone audiometry is often reported as the major, sometimes the only outcome parameter. In recent years, increasing efforts regarding quality assessment and assurance have been integrated in reconstructive middle ear surgery. Besides clinical and audiological outcome parameters after tympanoplasty, patient-related aspects such as HRQOL also play an increasing role.
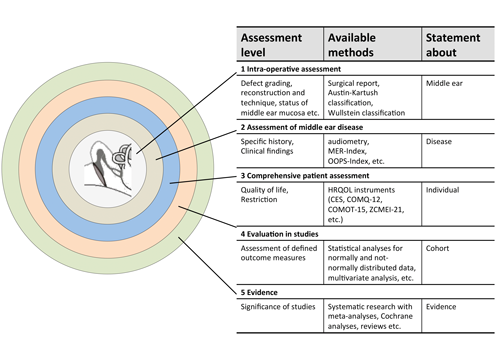
Figure 1. Assessment levels of middle ear disease and ear surgery (modified from Neudert and Zahnert, 2017 [1]). By triangulation of assessment instruments (available methods), a more exact statement can be made with an increasing number of methods. As of level 4, the results of single patients can be considered in the context of studies. The more detailed the characterisation performed on the previous levels; the more exact and valid the conclusions. (MER: middle ear risk [2]; OOPS: ossiculoplasty outcome parameter staging [3]; HRQOL: health-related quality of life; CES: chronic ear survey; COMQ-12: chronic otitis media questionnaire 12; COMOT-15: chronic otitis media outcome test 15; ZCMEI-21: Zurich chronic middle ear inventory). © Georg Thieme Verlag KG
Levels of disease description
The assessment of success or failure of reconstructive middle ear surgery for the individual patient seems to be easily communicable. But already when considering the patient as a whole individual, the description of the intraoperative site or the postoperative hearing result do not allow a comprehensive outcome statement. Therefore, additional assessment methods must be applied to define different quality dimensions as outcome parameters. Figure 1 presents a model of assessment levels of middle ear disease and / or surgery. Hereby, the triangulation of measurement instruments leads to complementary outcome parameters. Thus, the status description becomes broader with every step until the individual patient can be included in a study population at level 4. If the quality criteria are sufficient the results may be integrated in meta-analyses. In this regard, HRQOL instruments fill the gap between the individual’s subjective perceptions of disease-associated quality of life restriction and other diagnostic findings.
Validated and non-validated measurement instruments
In HRQOL assessment validated and non-validated measurement instruments are available. It is crucial for an implementation of HRQOL measurements in clinical and scientific routine to carefully choose a measurement method. Validated HRQOL measurements must be sharply delimited from patient interviews that inquire about symptoms and possible impairments by means of own item lists. These can exclusively contribute to the practical documentation of complaints. In order to find scientifically sound, reliable conclusions, the application of psychometric measurement instruments that meet all quality criteria (objectivity, reliability and validity) of a standardised measurement procedure, are essential. Beside statistical evaluation, only these allow a comparability of data and can contribute to the assessment of outcome parameters. Then different surgical techniques (e.g. open versus closed technique), preoperative disease variables (otorrhea) or specific patient’s attributed comorbidities (diabetes or revision surgery) can significantly influence the postoperative HRQOL.
Available HRQOL tools and their use
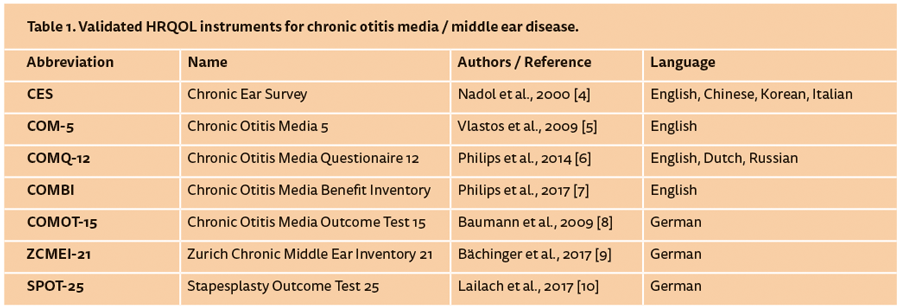
Scientifically, the HRQOL is understood as a multifactorial construct covering four dimensions: physical complaints, mental condition, functional impairment in daily life and impairment of interpersonal relationships. Those dimensions are analysed by means of targeted items from the patient’s point of view with regard to his specific disease. Since every disease has different symptoms, HRQOL measurements have to be based on disease-specific instruments. Most of the validated HRQOL questionnaires for the field of otology are available in English. Tympanoplasty as a surgical procedure is applied in a heterogenic group of middle ear diseases, and can therefore only be indirectly assessed in the context of a disease-specific evaluation. However, for chronic otitis media and cholesteatoma, as well as for surgical interventions, a number of validated instruments are available and summarised in Table 1. Additionally, the Hearing Satisfaction Scale (HSS), the Hearing Handicap Inventory for Adults (HHIA) and the (modified) Amsterdam Inventory of Auditory Disability and Handicap [(m)AIAD] are suitable to determine the restrictions associated with hearing loss in general. Generic measurement instruments, which are not disease-specific like the Short Form 36 (SF-36) or the Glasgow Benefit Inventory (GBI) can also be used. However, they might not be able to display the specific restrictions in middle ear diseases since they create more general statements.
“Since every disease has different symptoms, HRQOL measurements have to be based on disease-specific instruments.”
Language dependency and limitations
Using HRQOL measurement instruments, it must be taken into account that the questionnaires have to be validated in the according language. A questionnaire that has been developed and validated must not be simply translated by an investigator or clinician and applied without revalidation. The translation into another language may change the meaning of single items and thus even the overall statement of the test. Nonetheless, they may be used as an orientation for patient interviews. As a consequence, disease-specific HRQOL instruments must be translated and validated to compare results of studies conducted in non-English speaking countries. This was performed for the CES, the COM-5 and the COMQ-12.
In addition, the practical orientation of the test and the weighting of the relevant dimensions vary leading to inexactness of the assessment between the measurement instruments. The hearing impairment is certainly one important influencing factor in chronic otitis media, but it is perceived in different ways by different patients and individually weighted. The same is true for otorrhea. In summary, the number of questions about the impairments by the according symptom is decisive for the weighting and the differentiated assessment. These little differences and possible inaccuracies of the available HRQOL measurement instruments have to be recognised.
References
1. Neudert M, Zahnert. Tympanoplasty - news and new perspectives. Laryngo-Rhino-Otol 2017;96(S1):66-83.
2. Kartush JM. Ossicular chain reconstruction: capitulum to malleus. Otolaryngol Clin North Am 1994;27:689-715.
3. Dornhoffer JL, Gardner E. Prognostic factors in ossiculoplasty: a statistical staging system. Otology and Neurotology 2001;22:299-304.
4. Nadol JB Jr, Staecker H, Gliklich RE. Outcomes assessment for chronic otitis media: the Chronic Ear Survey. Laryngoscope 2000;110(3):32-5.
5. Vlastos IM, Kandiloros D, Manolopoulos L, Ferekidis E, Yiotakis I. Quality of life in children with chronic suppurative otitis media with or without cholesteatoma. Int J Pediatr Otorhinolaryngol 2009;73(3):363-9.
6. Phillips JS, Haggard M, Yung M. A new health-related quality of life measure for active chronic otitis media (COMQ-12): development and initial validation. Otol Neurotol 2014;35(3):454-8.
7. Phillips JS, Haggard M, Spencer H, Yung M. The Chronic Otitis Media Benefit Inventory (COMBI): Development and Validation of a Dynamic Quality of Life Questionnaire for Chronic Ear Disease. Otol Neurotol 2017;38(5):701-7.
8. Baumann I, Gerendas B, Plinkert PK, Praetorius M. Development and validation of the Chronic Otitis Media Outcome Test 15 (COMOT-15). Measurement of health-related quality of life in patients with chronic otitis media. HNO 2009;57:889-95.
9. Bächinger D, Röösli C, Ditzen B, Huber A. Development and validation of the Zurich chronic middle ear inventory (ZCMEI-21): an electronic questionnaire for assessing quality of life in patients with chronic otitis media. European Archives of Oto-Rhino-Laryngology 2016;273.
10. Lailach S, Schenke T, Baumann I, et al. Entwicklung und Validierung des Stapesplasty Outcome Test 25 (SPOT-25). HNO 2017 [Epub ahead of print] doi.org/10.1007/s00106-017-0389-x
CONCLUSION
-
A number of disease-specific HRQOL measurement instruments are available for middle ear diseases and provide additional outcome parameters in both the daily clinical routine focusing on the individual patient and in scientific studies.
-
The comparison of pre- and postoperative results of HRQOL measures provides additional parameters to evaluate and describe the outcome.
-
HRQOL instruments fill the gap between the individual’s subjective perceptions of disease-associated quality of life restriction and other diagnostic findings.
-
Therefore, they enhance the view on the individual patient and combine clinical and diagnostic findings.