Awareness of cochlear synaptopathy (‘hidden hearing loss’) is growing. Chris Plack gives us an introduction to the condition, defining it and reviewing recent research in humans and animals with respect to noise exposure.
The main cause of hearing loss is traditionally thought to be damage to the hair cells in the cochlea, particularly the sensitive outer hair cells that amplify the motion of the basilar membrane, and enhance its frequency tuning. However, experiments in mice and other rodents have shown that moderate noise exposure can cause a dramatic loss of synapses between inner hair cells and auditory nerve fibres without causing permanent hair cell damage, and without affecting threshold sensitivity [1].
Synapses are also lost due to ageing, in the absence of noise exposure. The nerve fibres affected are predominantly those with high thresholds that transmit information about sound components with moderate-to-high levels. Effectively, the information travelling up the auditory nerve to the brain is reduced, and this might have a significant impact on our ability to separate out and identify sounds that are important to us, such as speech.
Cochlear synaptopathy has been referred to as ‘hidden hearing loss’ [2] as it is assumed to occur without affecting audiometric sensitivity. However, some authors are now using ‘hidden hearing loss’ (incorrectly in my view) to refer to any listening difficulties in individuals with normal audiograms, leading to confusion in the literature. It is important when reading a scientific article or news item about hidden hearing loss to be clear what the authors are referring to when they use this term.
Human electrophysiological measures
The rodent experiments suggest that cochlear synaptopathy may be an undiagnosed cause of listening difficulties in humans, and may explain why some people with normal audiometric hearing have difficulties hearing speech in background noise. The fact that the noise exposure that causes substantial synaptopathy in mice (just 100 dB SPL for two hours) is less than the exposure many young people receive on a single visit to a night club or rock concert, has raised the fear that a large proportion of people may have damaged their hearing permanently through noise exposure. What used to be considered a harmless temporary threshold shift may be associated with permanent neural damage.

Figure 1. Is loud music doing more damage to our ears than we thought?
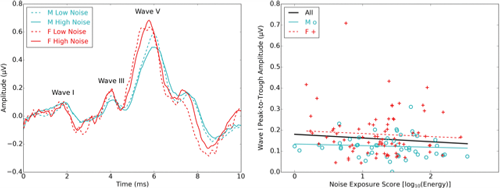
However, the evidence for hidden hearing loss in humans to date is mixed. In the rodent models, it is possible to count synapses directly post-mortem using histological techniques. In humans, we rely on indirect non-invasive measures, particularly wave I of the auditory brainstem response (ABR), an electrophysiological potential reflecting auditory nerve activity that can be recorded from electrodes placed on the scalp or in the ear canal. The assumption is that loss of synapses will be reflected by a reduction in wave I, as is the case in the rodent models. In a large study with 126 young adult participants, with normal audiograms but a wide range of self-reported lifetime noise exposures, we found no evidence for a reduction in wave I amplitude with increasing noise exposure [3]. This has been confirmed by two subsequent smaller studies from our laboratory, and in recent studies from other groups.
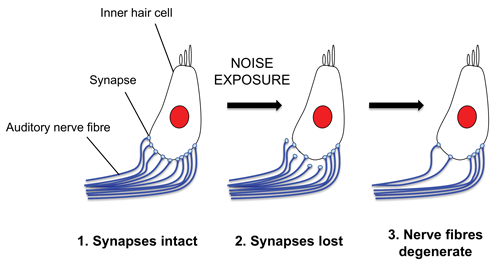
Figure 2. An illustration of the loss of synapses between inner hair cells
in the cochlea and auditory nerve fibres as a result of noise exposure.
Some research groups have reported an effect of noise exposure on wave I or similar electrophysiological measures, for people with hearing within the normal audiometric range. However, in these studies, the more noise exposed participants either had elevated high-frequency audiometric thresholds compared to the less exposed, or the very high-frequency thresholds (above 8 kHz) were not measured. This raises two possibilities: (1) synaptopathy was present in the more noise-exposed participants, and just tends to be accompanied by high-frequency audiometric loss; or (2) high-frequency loss of sensitivity directly affected the electrophysiological measures, potentially imitating the effects of synaptopathy. In any event, it is likely that humans are less susceptible to noise-induced hidden hearing loss than mice. It may be that only people with extreme noise exposures are in danger of substantial synaptopathy, and that this may always be associated with some permanent elevation in audiometric thresholds, particularly at very high frequencies.
Figure 3. The left-hand panel shows averaged auditory brainstem responses for young male (M) and female (F) adults with normal hearing, divided into groups with extreme low and high noise exposures. The right-hand panel shows the amplitude wave I of the auditory brainstem response (reflecting auditory nerve activity) as a function of lifetime noise exposure for 124 young adults. Data are replotted from Prendergast et al., 2017 (3).
Perceptual measures
Another possibility is that ABR wave I, and other indirect electrophysiological measures that have been tried, are not sufficiently sensitive to detect synaptopathy in humans. Wave I in particular shows high variability between individuals. An alternative approach is to examine whether noise exposure is related to perceptual deficits in people with normal audiometric hearing. Although there are some older reports that suggest this is the case, most of the recent studies, including our own, have found no relation between lifetime noise exposure and performance on perceptual tests for people with normal hearing, including the key measure of speech perception in noise [4].
Tinnitus has been associated with a reduction in wave I amplitude in listeners with normal audiograms [2], perhaps reflecting synaptopathy. Although a similar study in our laboratory did not replicate this result, we have shown that tinnitus with a normal audiogram is associated with lifetime noise exposure, suggesting that some forms of tinnitus may be associated with ‘hidden’ deficits produced by noise exposure [5].
Panic over?
So should we all stop worrying about hidden hearing loss? I think this would be a mistake for three reasons. First, we may not yet have found a sufficiently sensitive measure of synaptopathy in humans, and some of the negative results may be due to problems in measurement rather than an absence of synaptopathy. Second, even if it turns out that humans are much less susceptible to noise-induced synaptopathy than rodents, it is quite possible (likely even) that synaptopathy co-occurs with noise-induced hair cell damage, as revealed in the audiogram. Third, there is increasing direct post-mortem histological evidence that ageing in humans is associated with substantial synaptopathy, in addition to hair cell dysfunction and central neural dysfunction [1].
To understand the listening difficulties experienced by individuals with and without clinical hearing loss it may be necessary to go ‘beyond the audiogram’, to measure suprathreshold neural function in addition to hair cell function. We have a range of electrophysiological techniques that reveal individual differences in neural function for people with normal audiograms. The problem is that hair cell loss is also associated with a reduction in electrophysiological responses; the response of any part of the auditory pathway is dependent on earlier processing. A challenge then is to distinguish hair cell loss from synaptopathy and other neural dysfunctions, to provide a differential diagnosis in people with hearing difficulties. This knowledge may then be used to provide more effective fitting of hearing devices in the medium term, and eventually we may be able to repair the damaged structures using targeted drugs or stem cell treatments.
References
1. Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hearing Research 2017;349:138-47.
2. Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. Journal of Neuroscience 2011;31:13452-7.
3. Prendergast G, Guest H, Munro KJ, et al. Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hearing Research 2017;344:68-81.
4. Prendergast G, Millman RE, Guest H, et al. Effects of noise exposure on young adults with normal audiograms II: Behavioral measures. Hearing Research 2017;356:74-86.
5. Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ. Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hearing Research 2017;344:265-74.
Summary
-
Noise-induced cochlear synaptopathy (‘hidden hearing loss’), has been convincingly demonstrated in rodent models, in the absence of hair cell loss or threshold elevation
-
Evidence for noise-induced synaptopathy in humans is inconclusive at present
-
In humans, synaptopathy may always co-occur with a hair cell loss and audiometric threshold elevation, particularly at high frequencies
-
A challenge for diagnosis is to distinguish synaptopathy from hair cell loss, to inform management options.