‘New normal’ is another phrase that has become synonymous with this pandemic. In this article, consideration for ‘low-touch’ and ‘no touch’ audiological pathways are described for adoption as the ‘new normal’ for hearing healthcare.
The COVID-19 crisis has ushered in a new era in hearing healthcare that requires a radical rethinking of service delivery in audiology. Low- and no-touch services are now necessary for audiology patients – who are typically at the highest risk for COVID-19 morbidity and mortality due to their age – to access medical care. Fortunately, audiology is a technology-driven profession in terms of providing assessment and intervention, allowing unique opportunities to leverage remote and telehealth hearing care solutions.
While traditional diagnostic assessment to differentiate hearing loss due to ear disease, which has a low prevalence, requires a sound-treated environment and a comprehensive test battery, a less controlled environment with fewer tests could suffice for hearing aid fittings. This means that more than 95 percent of adults with hearing loss could be served using alternative low- or no-touch models of audiological care.
While very concerning, the ongoing pandemic also offers a unique opportunity to redefine and innovate how hearing healthcare professionals reach and serve patients in more responsive, efficient, and person-centered ways. Exploring alternative patient journeys is crucial to evolve audiology during the COVID-19 crisis and beyond.
A new era of hearing healthcare
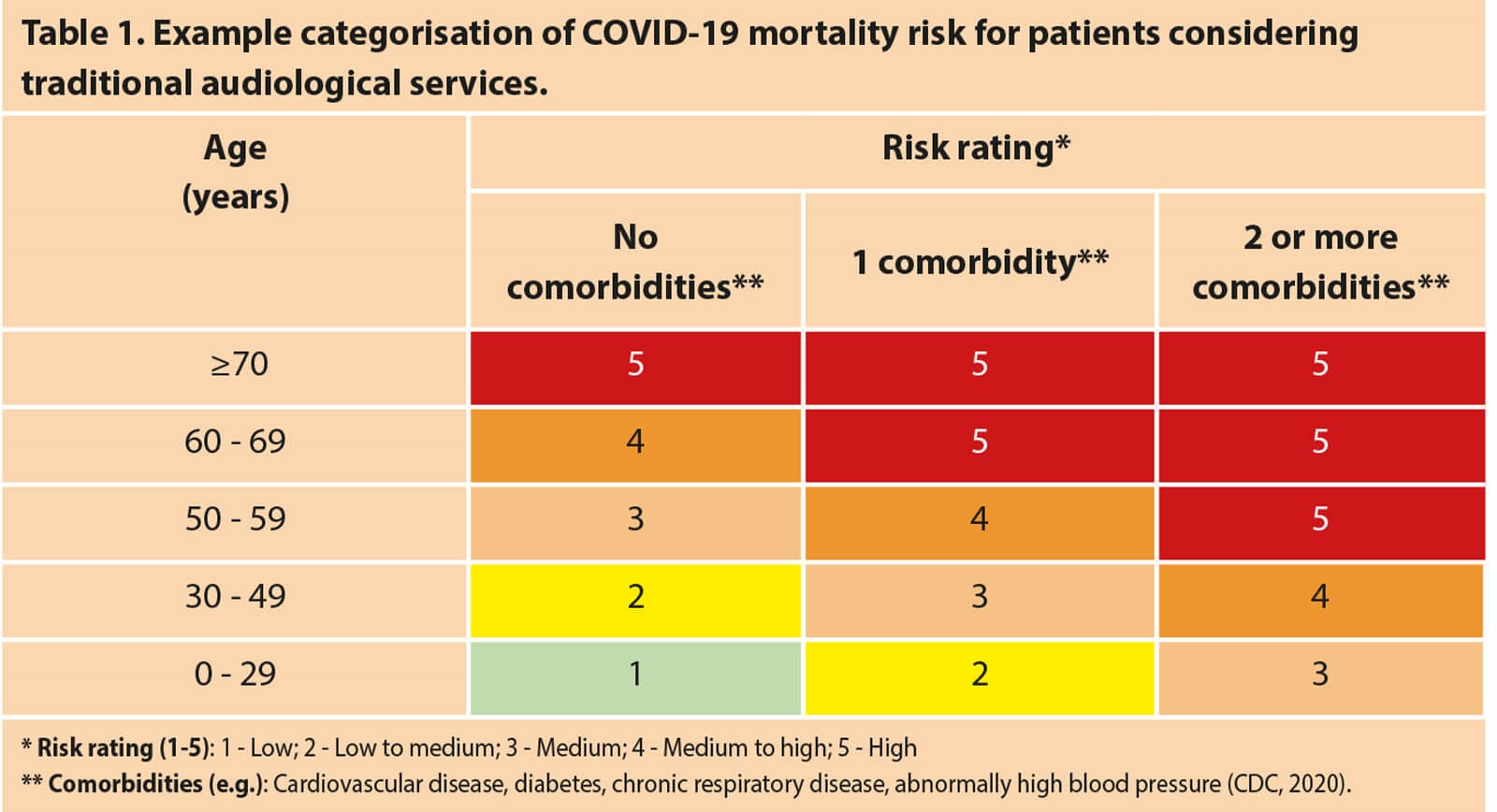
According to the Centers for Disease Control and Prevention (CDC) guidelines, audiological services pose a medium to high risk for COVID-19 infection, considering the proximity, test set-up, and length of appointments [1]. The fact that the majority of people who require audiology services (those over 65 years of age) are also the ones at the highest risk of COVID-19-related mortality and morbidity underscores the importance of reassessing how hearing care is delivered.
“More than 95 percent of adults with hearing loss could be served using alternative low- or no-touch models of audiological care”
Traditionally, audiological care has been a high-touch service with several face-to-face appointments in confined sound-treated spaces for initial assessments, hearing aid fittings, follow-up troubleshooting, and counselling. In this respect, how audiologists have been providing services to adults with hearing loss has remained very much the same over the past five decades. The sudden requirement for physical distancing, and even long-term lockdown recommendations for older adults, render this traditional audiological care pathway untenable at present.
Changing times require changes in care delivery
In the era of COVID-19, wherein low- or even no-touch services are necessary, audiological care needs to be responsive with alternative modes of service delivery. In our technology-driven field, unique opportunities to leverage connected solutions for remote and telehealth services exist. Where accessibility, convenience and efficiency have been the primary drivers of telehealth, COVID-19 has made it about safety first and foremost, considering the vulnerable profile of audiology patients.
Over the past several years, we’ve witnessed tremendous growth in digital hearing health care solutions, from web- and app-based screening to mobile audiometry, that has made decentralised community-based hearing care services possible [2-6]. These assessment options have typically relied on facilitators or assistants to guide patients through testing. Also, hearing aid manufacturers have been particularly good at including telehealth tools for remote device troubleshooting, counselling, fine-tuning, and tracking usage.
The challenge to audiologists and their patients is that tools for telehealth or remote care have typically been available in more expensive hearing aid options and, most importantly, serve existing patients only. While it is important to remotely support existing patients, the current situation requires urgent action to find alternative low- or no-touch mode to provide hearing assessments and hearing aids to new patients. This is important to ensure patients’ access to audiology services and, from a global perspective, keep audiology practice doors open and sustainable.
Low- or no-touch audiology options
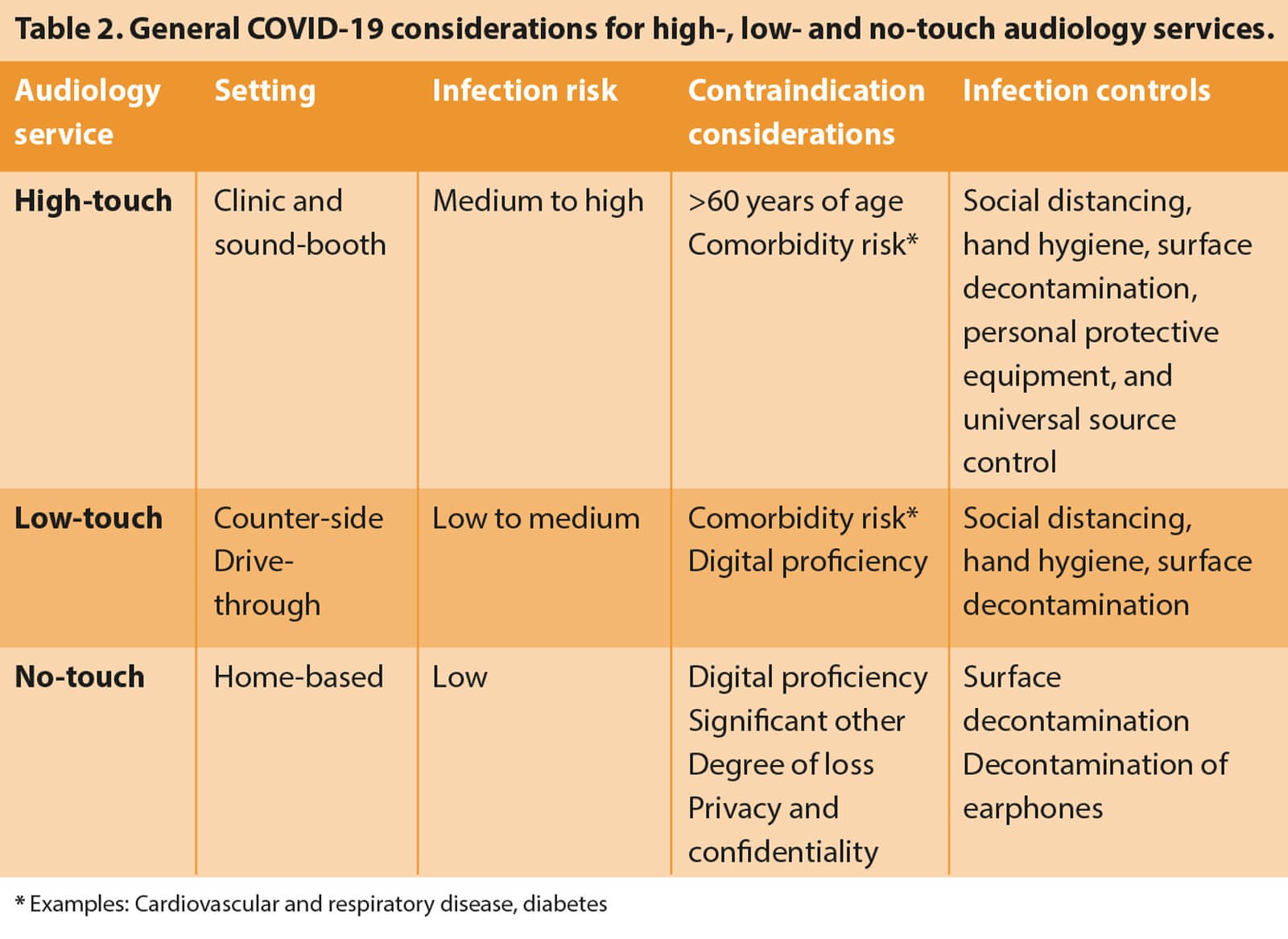
Patient risk profiles will need to be a driving factor in the mode of audiological services being provided (see Table 1 for an example risk categorisation grid). The risks posed by traditional audiology services to a population cohort that is most vulnerable to COVID-19 infection necessitate alternative low- or no-touch options for delivering audiological care [1]. In rethinking audiological care, it is important to differentiate the category and assessment purposes of the different adult patients we serve (Table 2). Audiological care for adults is generally directed to either (1) diagnostic assessments requiring a conventional sound-treated room to detect possible ear disease or (2) assessments for hearing aid fittings for which alternative point-of-care services may be suitable and, in the case of COVID-19, required.
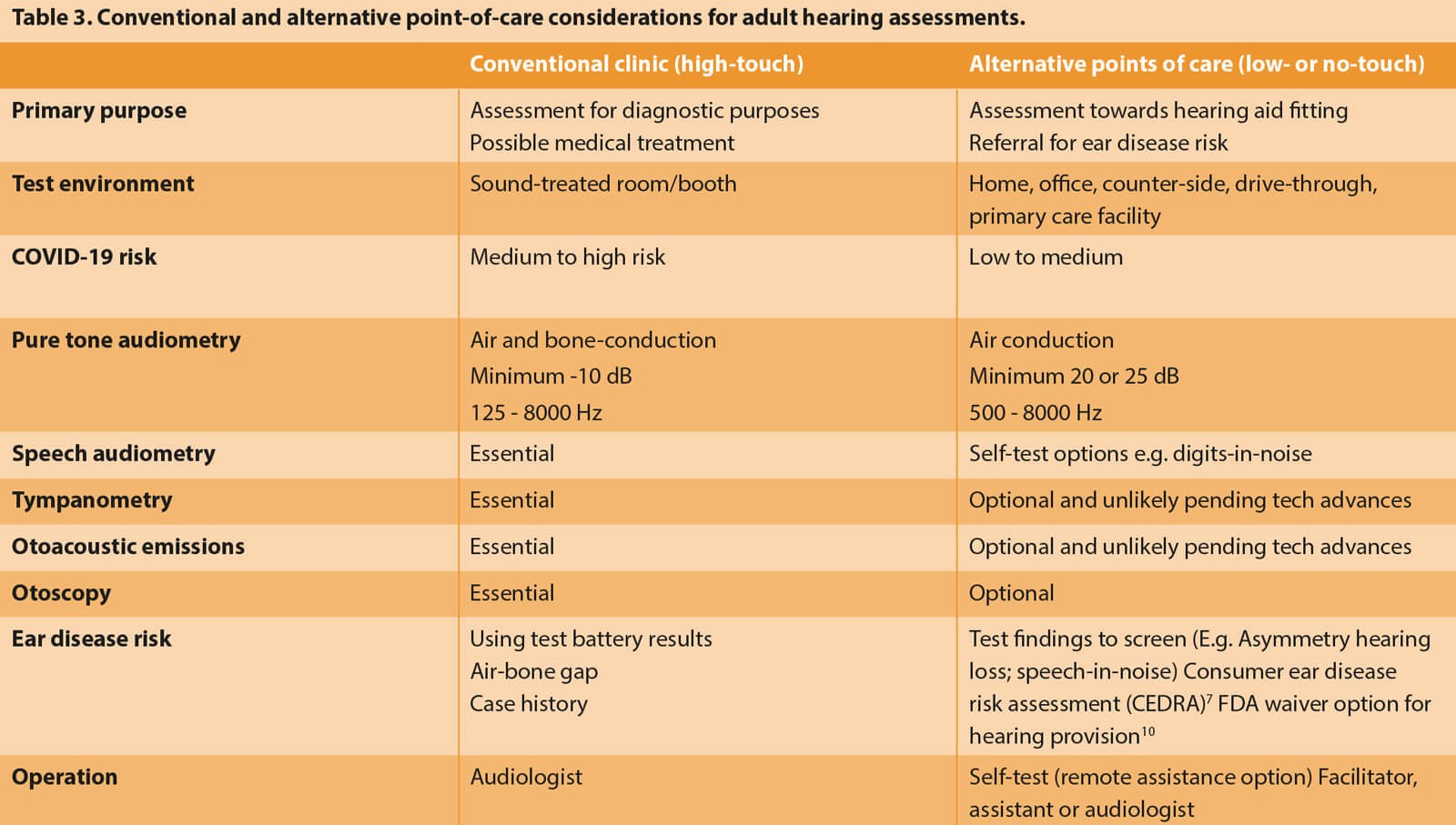
Audiological assessments, regardless of the purpose, have traditionally occurred in the same clinical sound-treated environment (Table 3). The reality, however, is that whereas a diagnostic assessment to confirm suspected ear disease requires a sound-treated environment and comprehensive test battery, a less controlled environment with fewer tests could suffice for hearing aid fittings. The diagnostic assessment for a patient with suspected ear disease requires air and bone conduction audiometry performed in a sound-treated room to ensure that reliable thresholds can be measured down to -10dBHL. Unoccluded bone conduction audiometry requires a maximum sound attenuation of at least a single-walled sound booth, and occluded testing leads to unreliable thresholds due to the occlusion effect. A patient with hearing loss who has no ear disease assessed for a hearing aid fitting may only require air conduction audiometry down to 20dBHL, which could occur without a sound booth.
The prevalence of ear disease or conductive loss in adult populations with hearing loss is very low, with reports varying between two to five percent [7,8]. Using conservative estimates, Zapala, et al estimated that the odds are 20:1 against encountering an ear condition that should be treated medically or surgically [9]. This means that more than 95 percent of adults with hearing loss could be served using alternative low- and no-touch models of audiological care.
Making low- and no-touch audiology work
The challenge in using low- and no-touch options is differentiating patients who require traditional clinic-based services, i.e. cases with suspected ear disease or conductive hearing loss, from those with sensorineural hearing loss who are likely candidates for hearing aids. Fortunately, some recent developments provide alternatives to conventional audiological testing, such as bone conduction audiometry and tympanometry, to identify the risk of ear disease. If a risk is detected for conductive hearing loss or ear disease, this small sub-group of patients can then be directed to high-touch audiology services in traditional settings where stringent COVID-19 guidelines must be employed to minimise infection risks (Table 2).
The Consumer Ear Disease Risk Assessment (CEDRA) is a validated questionnaire with a sensitivity of greater than 90 percent to detect ear disease [7]. Initially developed for consumer-based over-the-counter (OTC) and direct-to-consumer services, it is a valuable tool for making low- and no-touch audiology models work. The FDA prescribed questions to detect ear disease risk, and an asymmetric air conduction hearing loss could escalate referral to high-touch audiological and medical assessments [10,11]. Simple and quick test procedures can support the differentiation of patients at risk for conductive losses or ear disease. Recent work on antiphasic and diotic digits-in-noise testing has demonstrated the potential to differentiate conductive hearing loss by comparing speech recognition thresholds [2].
Air conduction tests in quiet and in noise have also previously been used to detect conductive hearing loss [12]. A recent study used pure tone and a digits-in-noise thresholds to differentiate conductive and sensorineural losses with sensitivity and specificity approximating 95 percent [13,14]. Detecting a conductive loss in this way during low- and no-touch services can direct at-risk cases to high touch audiology. Additional options include audiologist inspection of the patient’s external ear via close-up photographs by a family member in the home setting using a smartphone video-otoscope as part of a self-test kit [14,15].
A combination of these questionnaire-based and simple test procedures can provide a reliable triage directing patients to either traditional high-touch audiology services or low- or no-touch audiology alternatives (Table 3). A medical waiver option by the FDA for hearing aids could also serve as a way to mitigate risks when limited test results are available [10,16].
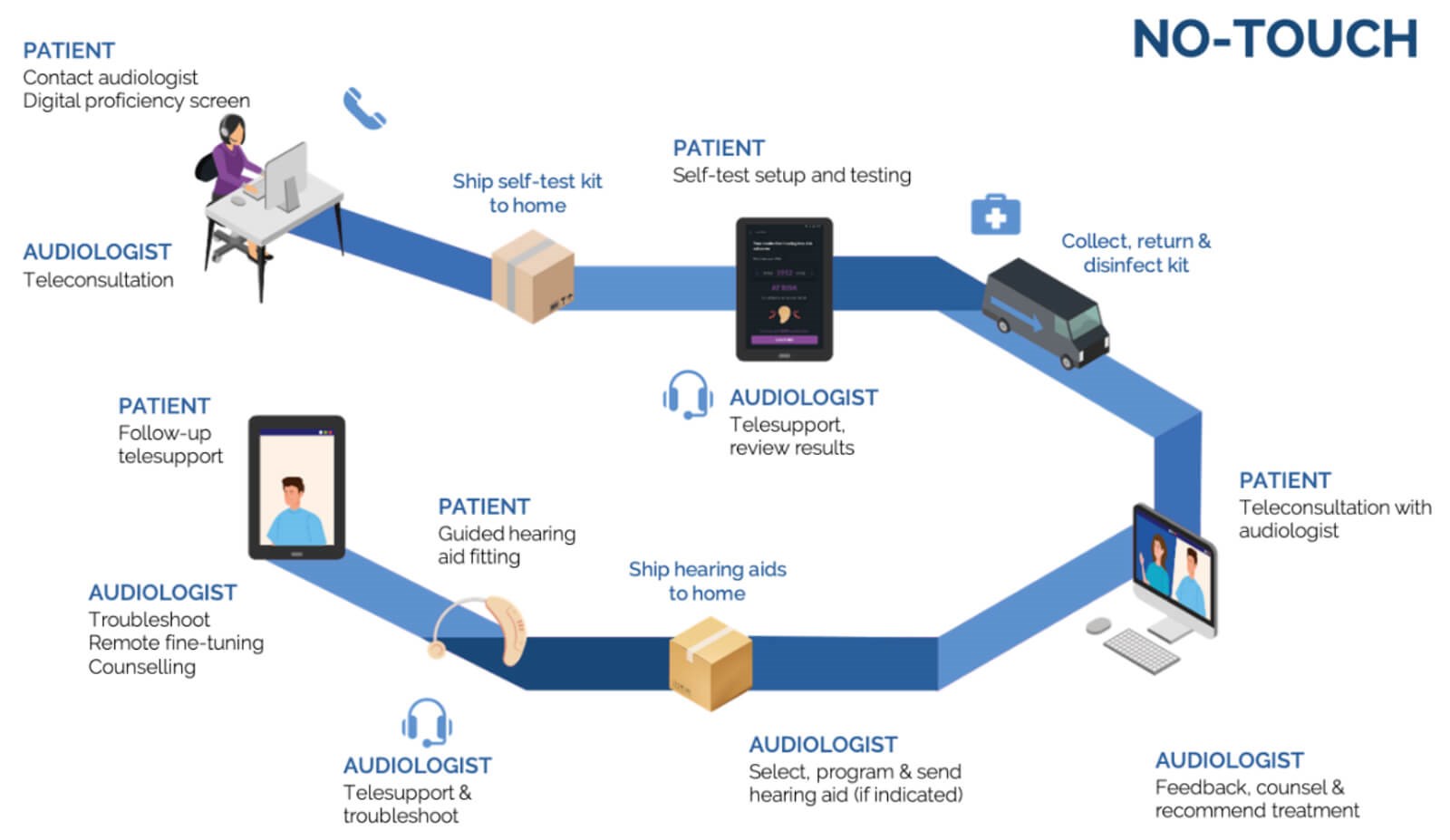
Figure 1. Example of no-touch audiology service journey.
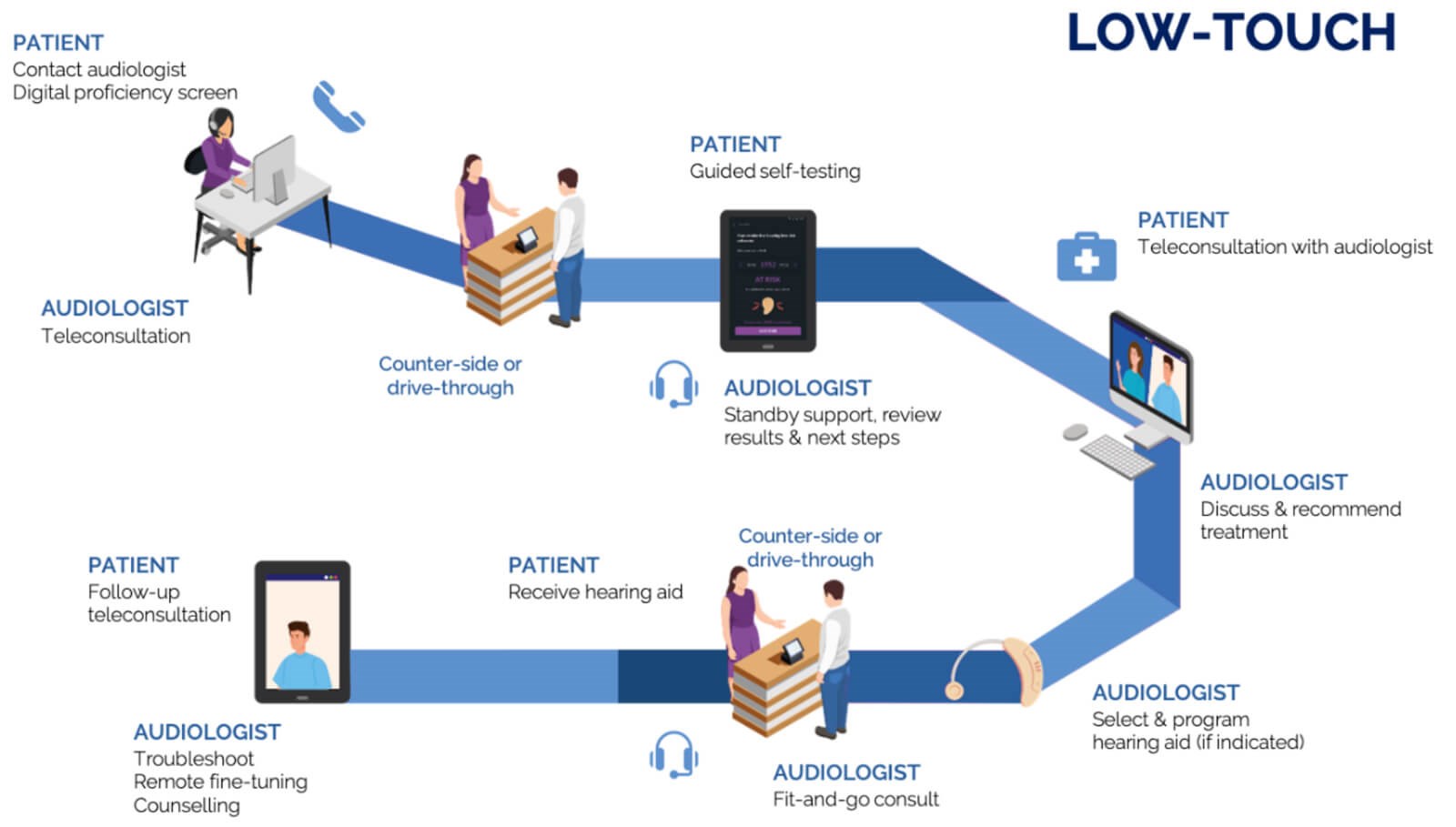
Figure 2. Example of low-touch audiology service journey.
Examples of low- and no-touch audiology
Moving outside of a conventional audiology clinic setting, at least outside of sound-treated rooms, could take the form of low-touch counter-side or drive-through services or even no-touch versions provided in patients’ homes (Tables 2 and 3). These home-based and counter-side services can come in the form of self-testing or facilitated assessments on digital devices with user-friendly step-by-step guidance and the option of real-time telehealth support from an audiologist. Figures 1 and 2 provide examples of possible no- and low-touch patient journeys and could also be envisaged as a hybrid of these two.
A recent example of a digital self-test kit released in response to the COVID-19 crisis aims to enable low- and no-touch audiological care [14]. The self-test kit allows self-guided tablet-based pure tone audiometry, speech-in-noise testing, ear risk assessment, and optional digital AI otoscopy. These types of solutions could facilitate initial assessments and tri-age adult patients assessed for hearing aid fittings with minimal face-to-face contact. Supported by teleconsultations throughout the assessment process (Figures 1 and 2), remote hearing aid troubleshooting and fine-tuning enable alternative low-touch models of care. While technology and connectivity are essential to these modes of care, they are there to support the role of the audiologist in providing person-centred care based on a relationship of mutual trust. Telecare tools like those provided by the Ida institute [17] offer a way to strengthen this partnership, along with information on a low- or no-touch patient journey.
To ensure the responsible use of low- and no-touch audiology options, audiologists must consider several aspects, including test setting, infection risk, contraindications, and infection control measures (Table 2). Digital proficiency levels are another possible factor that may prohibit no-touch and even low-touch options. Interestingly, a recent study suggests that digital proficiency may not limit people accessing alternative models of remote care [18]. It may, however, be valuable in determining the minimum levels of digital proficiency to determine whether no- or low-touch care options are appropriate. Fortunately, there are well-validated and brief questionnaires that clinicians can use to quickly screen for mobile device and computer proficiency [18-20].
The way forward
The COVID-19 crisis is likely to continue as a pervasive global influence persisting well into 2021 and beyond [21,22]. While it is posing a tremendous threat to the viability of traditional audiological services, it also creates the impetus to rapidly deploy and scale innovative digital and telehealth approaches in response to a changing landscape.
There are always challenges when expanding existing ways of service provision including, in this case, restrictions in several states that still require bone conduction audiometry, speech reception thresholds, and discrimination testing for hearing aid dispensing. The urgency to have audiological care in low- and no-touch settings, the pending OTC regulations, and FDA medical waivers for hearing aid provision make a strong case for regulatory bodies to adjust such requirements. Precedence exists for making allowances during the COVID-19 crisis as seen by the US Department of Health & Human Services making special allowance for the use of several videoconferencing platforms that were not previously authorised [23].
It is up to us as audiologists, professional associations, including AAA, ADA, and ASHA, industry, and even patient organisations like HLAA to drive lobbying and advocacy efforts for required allowances by legislators, third-party payers and healthcare insurers. For the sake of our patients and the continuation of our profession under current circumstances, changes are absolutely necessary.
The COVID-19 crisis is a watershed moment. While the threat is very real, it offers a unique opportunity to redefine and innovate how we reach and serve our patients in more responsive, efficient, and person-centred ways. As a profession reliant on technology, and as the world enters the fourth industrial revolution, timing has never been better for audiology to have its revolution in the provision of care.
Article reprinted from The Hearing Journal June 2020 issue, pages 20-24.
https://journals.lww.com/thehearingjournal/
Fulltext/2020/06000/Making_Audiology_Work
_During_COVID_19_and_Beyond.1.aspx
References
1. Centers for Disease Control and Prevention. (2020) Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19).
www.cdc.gov/coronavirus/
2019-ncov/hcp/guidance-risk
-assesment-hcp.html
2. De Sousa K, Swanepoel D, Moore D, et al. Improving sensitivity of the digits-in-noise test using antiphasic stimuli. Ear and Hearing 2020;41(2):442-50.
3. Ratanjee-Vanmali H, Swanepoel D, Laplante-Lévesque A. Patient uptake, experience and satisfaction using web-based and face-to-face hearing health services: Process evaluation study. Journal of Medical Internet Research 2020;22(3):e15875.
4. Swanepoel D. eHealth technologies enable more accessible hearing care. Seminars in Hearing 2020;41(02):133-40.
5. Swanepoel D, Smits C, De Sousa K, Moore, D. Mobile apps to screen for hearing loss: Opportunities and challenges. Bulletin of the World Health Organization 2019;97(10):717-8.
6. Van Wyk T, Mahomed-Asmail F, Swanepoel D. Supporting hearing health in vulnerable populations through community care workers using mHealth technologies. International Journal of Audiology 2019;58(11):790-7.
7. Klyn N, Kleindienst Rober S, Bogle J, et al. CEDRA: A tool to help consumers assess risk for ear disease. Ear and Hearing 2019;40(6):1261-6.
8. Hoff M, Tengstrand T, Sadeghi A, et al. Auditory function and prevalence of specific ear and hearing related pathologies in the general population at age 70. International Journal of Audiology 2020;1-12.
9. Zapala DA, Dhar S, Nielsen D, et al. (2015) Managing disease risk in hearing healthcare delivery [PowerPoint].
https://phhp-slhs.sites.medinfo.ufl.
edu/files/2012/11/UF-IOM-DISEASE
-RISK-PRESENTATION-.pdf
10. U.S. Food and Drug Administration. (2019). CFR - Code of Federal Regulations Title 21.
www.accessdata.fda.gov/scripts/cdrh/
cfdocs/cfCFR/CFRSearch.cfm?fr=801.420
11. Kesser BW. Clinical thresholds for when to test for retrocochlear lesions: Con. Archives of Otolaryngology-Head & Neck Surgery 2010;136(7):727-9.
12. Convery E, Keidser G, Seeto M, et al. Identification of conductive hearing loss using air conduction tests alone: reliability and validity of an automatic test battery. Ear Hear 2014;35(1):e1-8.
13. De Sousa KC, Smits C, Moore DM, et al (in press). Pure-tone audiometry without bone-conduction thresholds: using the digits-in-noise test to detect conductive hearing loss. International Journal of Audiology.
14. hearX group. (2020). COVID-19 hearing care solution, hearX self-test kit.
www.hearxgroup.com/blog/
hearX-self-test-kit.html
15. Yancey KL, Cheromei LJ, Muhando J, et al. Pediatric hearing screening in low-resource settings: Incorporation of video-otoscopy and an electronic medical record. International Journal of Pediatric Otorhinolaryngology 2019;126:109633.
16. U.S. Food and Drug Administration. (2016). FDA takes steps to improve hearing aid accessibility.
www.fda.gov/news-events/press-announcements/
fda-takes-steps-improve-hearing-aid-accessibility
17. Ida institute (2020). Telecare tools.
https://idainstitute.com/tools/telecare/
18. Ratanjee-Vanmali H, Swanepoel D, LaPlante-Levesque A. (Submitted). Digital proficiency is not a significant barrier for acquiring hearing aids and support services with an online and face-to-face model. American Journal of Audiology (Submitted).
19. Boot WR, Charness N, Czaja SJ, et al. Computer proficiency questionnaire: Assessing low and high computer proficient seniors. Gerontologist 2015;55(3):404-11.
20. Roque NA, Boot WR. A new tool for assessing mobile device proficiency in older adults: The mobile device proficiency questionnaire. Journal of Applied Gerontology: The Official Journal of the Southern Gerontological Society 2018;37(2):131-56.
21. Adam D. Special report: The simulations driving the world’s response to COVID-19. Nature 2020;580(7803):316-8.
22. World Health Organization. (2020). Public statement for collaboration on COVID-19 vaccine development.
www.who.int/news-room/detail/13-04-2020
-public-statement-for-collaboration-on
-covid-19-vaccine-development
23. U.S. Department of Health & Human Services. (2020). Slow the spread of the coronavirus.
www.hhs.gov
24. Centers for Disease Control and Prevention. (2020). Coronavirus Disease 2019 (COVID-19): Groups at Higher Risk for Severe Illness.
www.cdc.gov/coronavirus/2019-ncov/
need-extra-precautions/
groups-at-higher-risk.html






