Understand us; where do we begin? In this article the authors’ introduce a project that may uncover that our personalities and traits are a product of the interconnected wiring within our brain. The team discusses the Human Connectome Project and how this ambitious project plans to use advances in science and technology to unlock the brain’s interconnections which ultimately make us, us!
Ever wondered what makes you ‘you’ – and not someone else? Why do you exhibit the mannerisms that characterise you, why you can be anxious or fractious or relaxed and laid back, feel passionately about food, great wine or music (or not!), or why you may have lofty liberal or cool conservative inclinations?
According to Dr Francis Collins, Head of the National Institutes of Health in the USA, it is likely to be the way we are each individually wired – that ‘symphony’ of neural connection within our brains [1]. It’s probably no exaggeration to say that we are our connectomes! A connectome is a network map of effective synaptic connections and neural projections that comprise a nervous system and that shape its global communicative functions.
“The project hopes to deliver a revolutionary dynamic picture of the brain that, for the first time, will show how individual cells and complex neural circuits interact in both time and space.”
The Human Connectome Project (HCP, http://www.humanconnectomeproject.org/), launched by President Obama in 2013 as part of his Brain Initiative (http://www.braininitiative.nih.gov/), is a project that aims to map out the neural connections of the human brain in their entirety. The project hopes to deliver a revolutionary dynamic picture of the brain that, for the first time, will show how individual cells and complex neural circuits interact in both time and space. It will thus reveal how the brain enables us to record, process, utilise and retrieve massive quantities of data, all at the speed of thought and, most importantly, how disease processes affect this connectivity. The HCP aims to provide an unparalleled compilation of neural data, an interface to graphically navigate it and the opportunity to achieve never before realised conclusions about the living human brain. And what is so exciting is that the auditory system is pivotal to the structure and function of the human connectome (Figure 1).
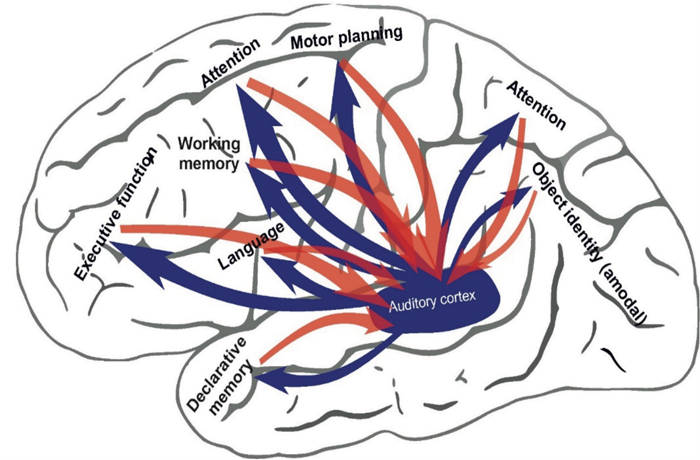
Figure 1. A schematic representation of the auditory system within the brain’s connectome.
The HCP project is wildly ambitious. Just reflect on the challenge: the human brain has about 100 billion neurons, each with about 10,000 or more connections; thus the number of neural connections in a typical brain can range between 100 and 500 trillion! To put this in perspective, the Human Genome Project required the mapping of a mere 2.9 billion base pairs which, at the time, stretched technological capability. The first organism to have its connectome unravelled was the simple round worm (C. Elegans) – this creature which measures no more than 1mm has only 300 neurons and a total of 7,000 connections – yet it took scientists about a decade to complete the task.
However, major advances in informatics and computational science are making the seemingly impossible HCP project a reality in the foreseeable future. The approach is multifaceted with some scientists using advanced imaging methodologies (e.g. diffusion tensor imaging – a technique that does not directly image neurons but uses mathematical constructs to predict their most likely trajectory) while others are choosing to use molecular and behavioural approaches – all techniques requiring advanced computational power.
Even though some technological challenges have yet to be overcome, the perspective of the highly interconnected brain also helps to better understand the consequences of sensory diseases. Given that brain subsystems are highly interconnected, loss of one system must have consequences beyond the affected system.
Furthermore, each sensory system is unique in its contribution to cognition: vision is a champion in spatial localisation and helps to organise information in space and with regard to a spatial reference. Hearing excels in ordering events in time. The brain uses the sensory systems as an entrance gate for information, but also as a reference frame for ordering and storing information. Loss of one system therefore leads not only to compensatory supranormal, but also subnormal performance of the remaining functions, and influences the outcome of future therapy. This is particularly true when sensory loss affects the brain during a highly-vulnerable state, for instance, during early childhood. In childhood, juvenile neurons can easily modify their mutual interconnections.
“The study makes aware that the brain is a large connected network, a connectome, and hearing loss during childhood a disease involving the ear, but also the whole brain – it is a connectome disease.”
Researchers have now highlighted the variable neurocognitive sequelae of hearing loss in early childhood and how these influence outcome following cochlear implantation [2]. Much has been learned about such non-auditory adaptations from deaf animal models as well as through undertaking neurocognitive investigations on deaf children, with and without cochlear implants. In particular, deafborn children exhibited alterations in cognitive function related to timing and sequencing of events. These deficits were not observed in all such children: high inter-individual variability was noted, which fits with the wide range of outcomes typically observed in clinical practice.
White Matter Fibers, HCP Dataset view from below.
White Matter Fibers, connecting gyri and hemispheres.
Courtesy of the USC Laboratory of Neuro Imaging and Athinoula A. Martinos Center for Biomedical Imaging, Consortium of the Human Connectome Project - www.humanconnectomeproject.org
The authors suggest that such connectome adaptations to deafness are highly individual, depending on the particular strategy used to cope with the hearing loss and thus have important clinical implications. The authors suggest a battery of age-appropriate cognitive testing to identify these individual cognitive adaptations before or soon after therapy of hearing has been initiated.
The study not only connects authors around the globe with the focus on deafness, it connects science across many disciplines: basic neuroscience, cognitive psychology and linguistics, audiology and otolaryngology, all contributing to the view of sensory loss being more than loss of hair cells. The study makes aware that the brain is a large connected network, a connectome, and that hearing loss during childhood is a disease involving the ear, but also the whole brain – it is a connectome disease. Whether cognitive training can resolve the issues remains a research question for the future.
Deafness as a connectome disease is a welcome concept for all hearing professionals, since it offers a platform to evaluate and manage the wide range of outcomes seen in everyday clinical practice. It also paves the way for individualised and personalised medicine, focusing on the neurocognitive characteristics of the individual child and not being driven by group data.
References
1. http://directorsblog.nih.gov/
the-symphony-inside-your-brain/
2. Kral A, Kronenberger WG, Pisoni DB, O’Donoghue GM. Neurocognitive factors in sensory restoration of early deafness: a connectome model. Lancet Neurol 2016; [Epub ahead of print] DOI:
http://dx.doi.org/10.1016/S1474-4422(16)00034-X
Declaration of Competing Interests: None declared.







