Ecological momentary assessment (EMA) is gaining momentum in the world of connected hearing healthcare and real-world assessment. Barbra Timmer explains how EMA will play a key role in transforming the information that clinicians use in decision-making and measuring outcomes.
Did you know that the pure-tone audiogram is almost 100 years old [1]? Despite its age, the pure-tone audiogram remains a cornerstone of audiology assessment and it drives clinicians’ decision making in many audiology practices, even today.
This is surprising to a certain extent, as research has shown it is not particularly sensitive to giving insight into how an adult experiences their hearing impairment [2] or in predicting success with rehabilitation options such as hearing aids [3]. Self-report disability questionnaires and surveys can be used to augment audiometric testing and provide clinicians better predictors for hearing aid √ptake, use and satisfaction but only if the survey questions are relevant to the individual [4]. Similarly, hearing aid success is best measured in listening situations that are real-world and relevant to the patient.
To record the real-world experiences of individuals with hearing impairment, the use of ecological momentary assessment (EMA) has gathered momentum in hearing science. EMA, also called ambulatory assessment or experience sampling, requires individuals to complete self-report surveys several times a day, as select experiences or events occur. In audiology, a typical EMA survey may include questions about the specific listening situation (for example, listening activity or intent), the acoustic environment (for example, room and speaker characteristics) and a rating of hearing performance (for example, a rating of speech understanding or listening effort).
As the surveys are completed during the listening event, it does not require patients to either generalise or remember the situation, thereby reducing recall bias. EMA can involve the use of paper diaries but compliance, convenience and accuracy has been shown to be better when using EMA applications (or apps) on electronic devices such as smartphones to collect surveys. Patients can elect to trigger a survey themselves or respond to a survey after a smartphone reminder. EMA has been shown to be valid and reliable and has been utilised in audiology research to investigate patients with tinnitus, the communication difficulties of adults with hearing impairment in different listening situations and hearing aid benefit and performance differences [5].
For future clinical applications, EMA may play a role in transforming the information that clinicians use in decision-making and measuring outcomes. As EMA is highly individualised, it can provide insights that are typically not captured today. For example, the International Outcome Inventory for Hearing Aids (IOI-HA) measures hearing aid benefit with the question: “Think about the situation where you most wanted to hear better, before you got your present hearing aid(s). Over the past two weeks, how much has the hearing aid helped in those situations?”. This requires the patient to generalise hearing aid benefit across all the distinct listening situations they encountered during those past two weeks. The EMA approach would be to include “how much are your hearing aid(s) helping you in your current listening situation?” and can include questions that define ‘helping’ and hearing aid benefit more specifically. A recent EMA pilot study showed that for 10 older adults with mild hearing loss, a short two-week trial with hearing aids showed benefit in terms of speech understanding, but more so in terms of reduced listening effort, feeling less hampered by hearing difficulties and increased enjoyment of listening situations [6] (see Table 1).
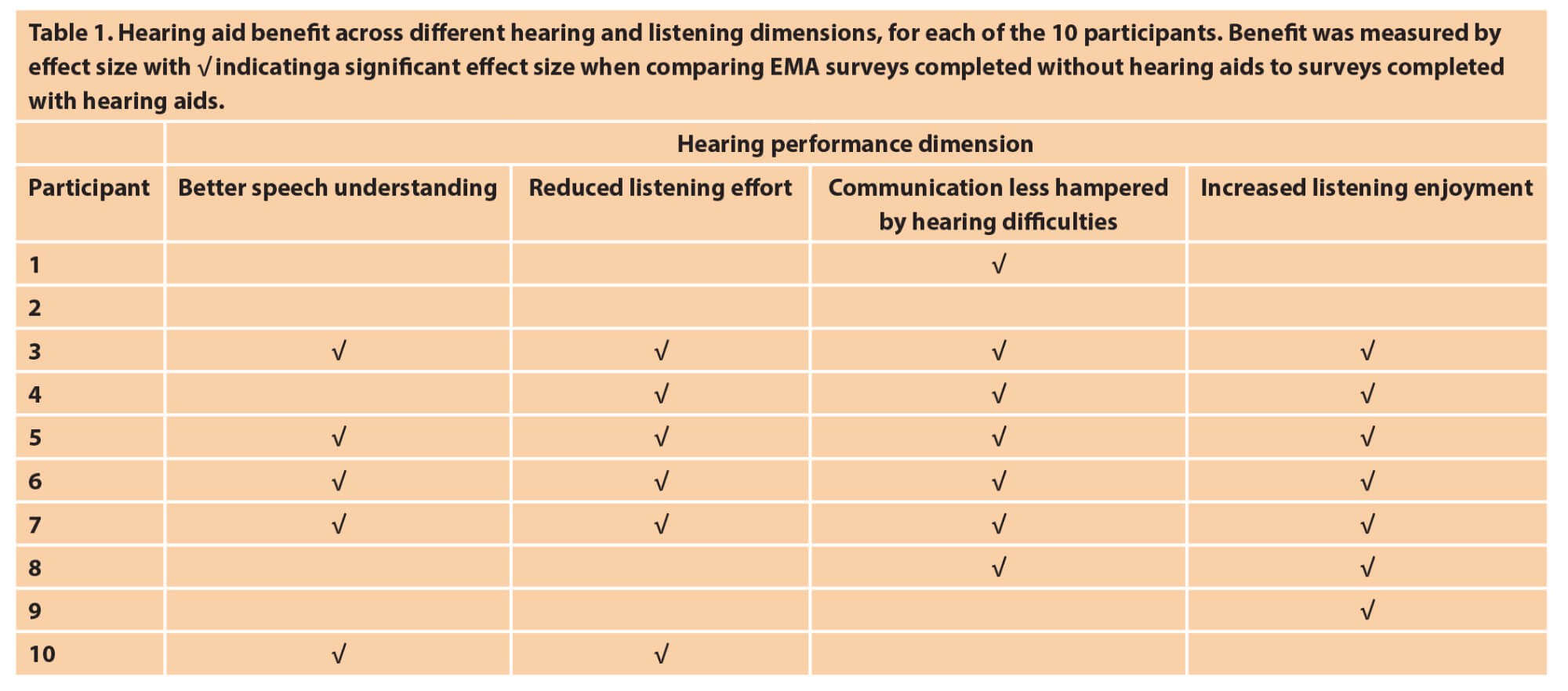
The study also compared the individual EMA outcomes with the participants’ IOI-HA information and while the two outcome measures are not designed to measure the same concepts, the study proved that the different dimensions that can contribute to hearing aid benefit can be measured using an EMA approach and this information can complement traditional outcome measures.
The information in Table 1 demonstrates how EMA data in future could be presented in a manner that is relevant and valuable for clinical decision-making during the rehabilitation process. Participant 2 may not (yet) be a candidate for hearing aids as no benefit was reported in the EMA surveys. The information from participant 8 could alert their clinician to fine tune or programme their hearing aids to obtain more benefit in terms of speech understanding (such as improving settings or programmes for speech in noise situations) and listening effort (such as increasing noise canceller settings).
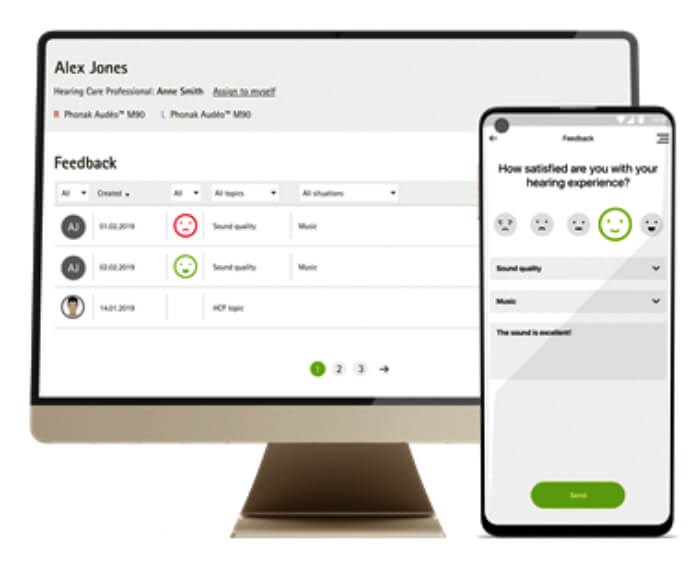
Figure 1. One example of an in-situ hearing aid satisfaction rating app and clinician dashboard.
While the EMA apps used in hearing science have been largely designed for research purposes, there are already a number of apps available today that allow hearing aid wearers to record their satisfaction with their devices in real-world experiences in a less detailed manner. The myPhonak smartphone app (see Figure 1), for example, includes a Hearing Diary that allows wearers to use a simple satisfaction rating scale and listening situation descriptor to note their experiences with their devices in real-world situations. They can also use the app to send a message to their clinician, who can view the patients’ feedback on a dashboard, answer messages and take further action if required. That action may be reprogramming of the hearing aids or further counselling on the use of different hearing aid settings and programmes.
If EMA apps evolve from research into fully-fledged clinical tools, they can be expected to become a strong tool for hearing care clinicians to gain further insight and provide individualised, person-centred care during the audiology rehabilitation process.
References
1. Jerger J. The evolution of the audiometric pure-tone technique. Hearing Review 2018;25(9):12-8.
2. Timmer BHB, Hickson L, Launer S. Adults with mild hearing impairment: Are we meeting the challenge? International Journal of Audiology 2015;54(11):786-95.
3. Hickson L, Meyer C, Lovelock K, et al. Factors associated with success with hearing aids in older adults. International Journal of Audiology 2014;53(Suppl 1):S18‑27.
4. Knudsen LV, Öberg M, Nielsen C, et al. Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: A review of the literature. Trends in Amplification 2010;14(3):127-54.
5. Timmer BHB, Hickson L, Launer S. Ecological momentary assessment: Feasibility, construct validity and future applications. American Journal of Audiology 2017;26(3S):436-42.
6. Timmer BHB, Hickson L, Launer S. Do hearing aids address real-world hearing difficulties for adults with mild hearing impairment? Results from a pilot study using ecological momentary assessment. Trends in Hearing 2018;22.
Declaration of Competing Interests: Dr Barbra Timmer is employed as Senior Scientist by Sonova AG, Switzerland.






