One of the most common causes of tinnitus is noise exposure, be that either cumulative day-to-day exposure over a lifetime or experience of acute noise trauma such as a loud concert or shooting incident. Observational data indicate that up to 40% of tinnitus patients may report noise-related activities as a causal factor for their tinnitus, with typical reports being exposure to a long-duration noise, an explosion or a brief intense noise (specialist tinnitus clinic in Portland, Oregon, USA [1]).
Models of noise trauma have established that chronic subjective tinnitus is maintained by neural processes happening in the brain rather than just resulting from peripheral events within the ear itself. Tinnitus is often considered to be a neuroplastic response to hearing loss because otologic conditions, especially noise-induced hearing loss and presbyacusis, present major risk factors for its onset. In this short review, I present a selection of recent studies that employ brain imaging techniques to examine questions about tinnitus-related neuroplasticity.
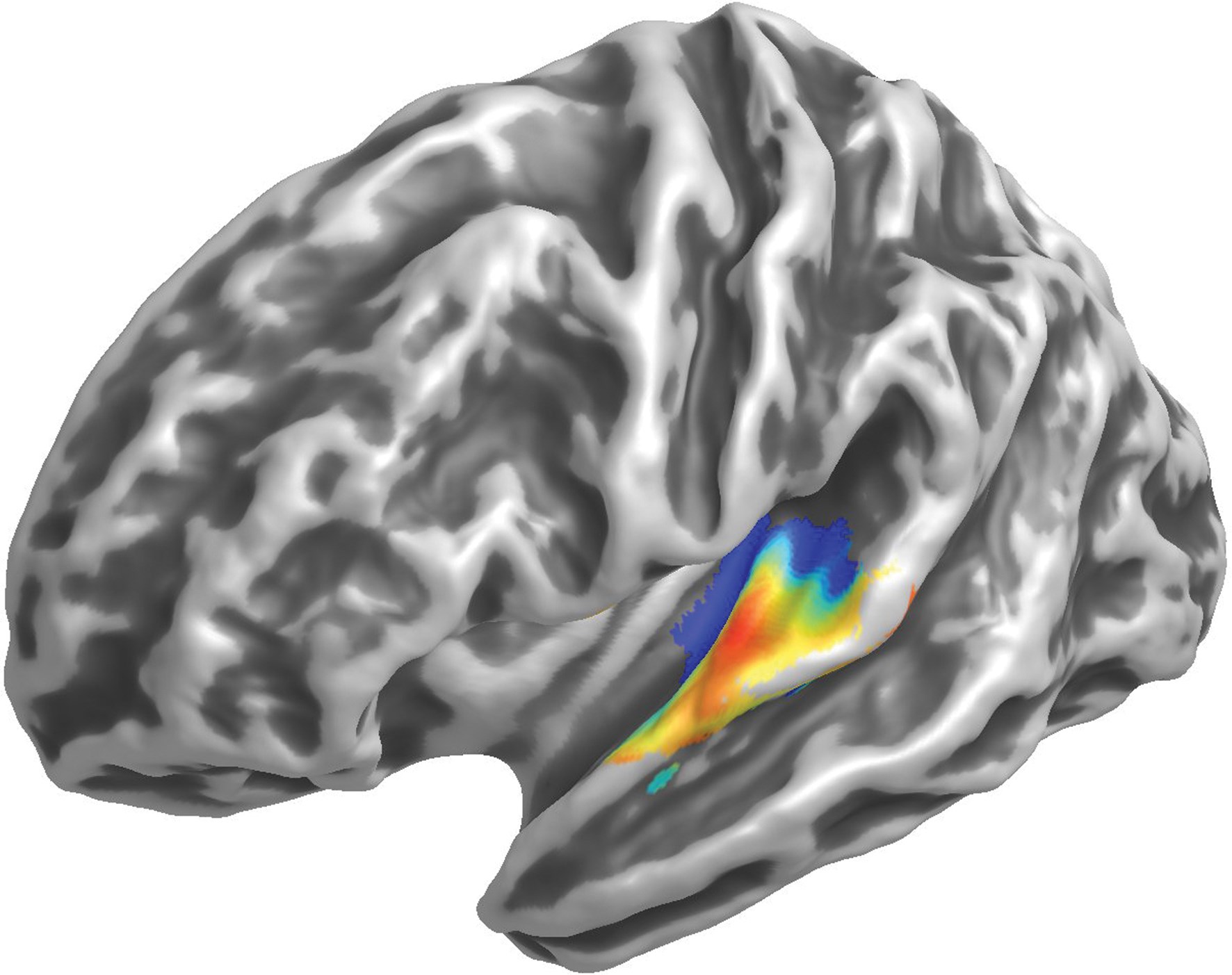
Figure 1: An example of the orderly frequency gradient around human primary auditory cortex in response to tones presented at different frequencies. The colour scale proceeds from blue (low frequencies) to red (high frequencies) and the view shows the left hemisphere.
Frequency reorganisation in the vicinity of primary auditory cortex
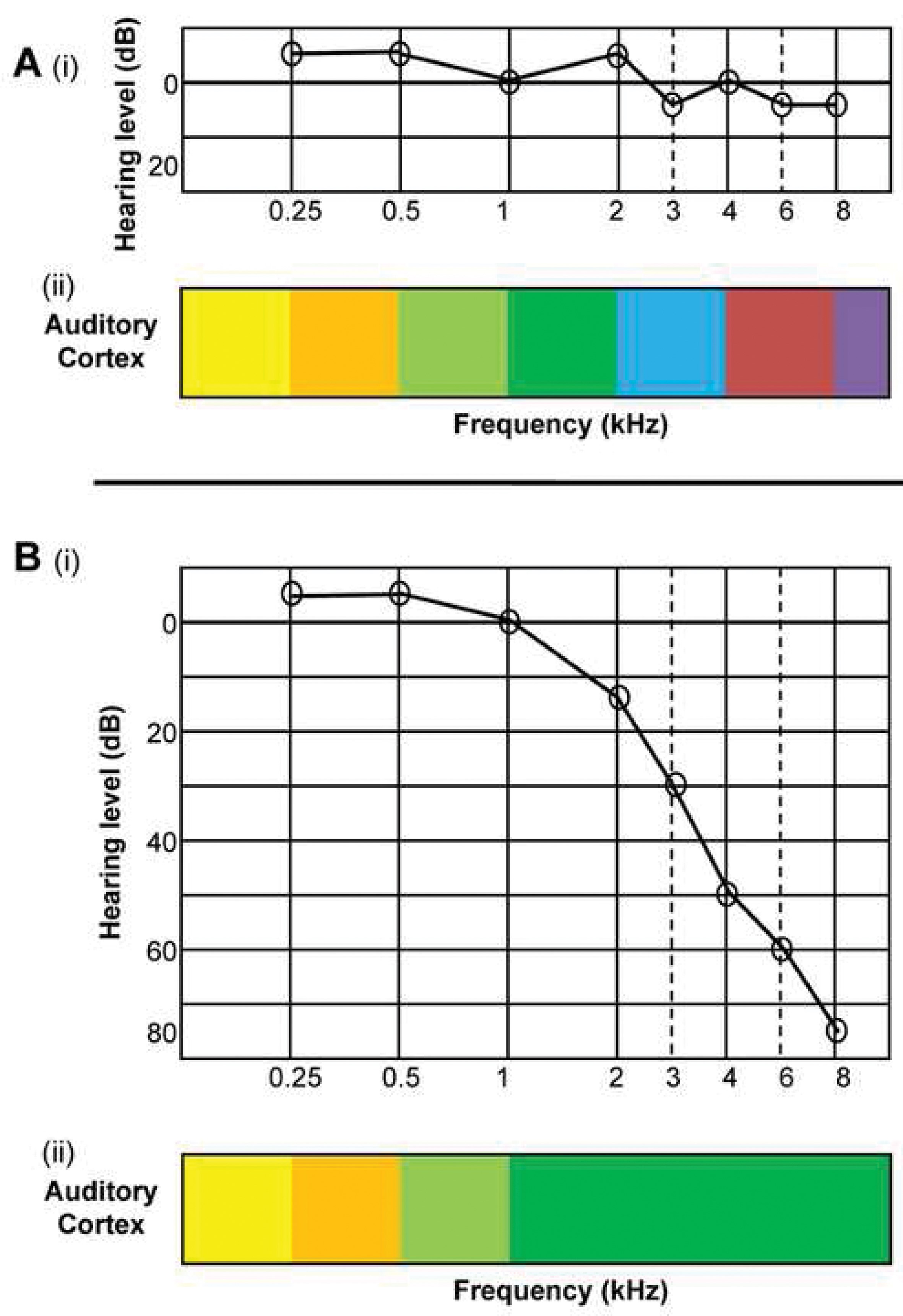
One consequence of high-frequency hearing loss revealed by animal models is that temporary or permanent damage to sensory cells in the inner ear triggers over-compensatory mechanisms that may be responsible for tinnitus. A well-known study by Rajan and Irvine showed that cortical neurons in the hearing loss region begin to respond preferentially to sound frequencies at the audiometric edge, such that edge frequencies come to be over-represented in the frequency map of the primary auditory cortical field [2]. Rather than there being the normal orderly progression (gradient) of responses across the frequency spectrum, they observed a preponderance of neurons that preferentially responded to the edge frequency. Figure 1 illustrates the normal cortical map associated with clinically normal hearing (panel A) and the reorganised map associated with high frequency hearing loss (panel B).
“Functional neuroimaging has the potential to be a very powerful window into the tinnitus brain. That it has not yet achieved its potential reflects more on a limitation of the creativity of the scientists than of the scientific method.”
In humans, electroencephalography (EEG) has been used to search for the same effect. Researchers in Germany used the 40-Hz auditory steady-state response to assess representation of frequency gradients around primary auditory cortex in people with chronic subjective tinnitus and hearing impairment [3]. These patterns were reported to be ‘degraded’ compared with those of normal hearing controls. This sort of macroscopic reorganisation is not necessary for tinnitus, however. Using functional magnetic resonance imaging (fMRI), a group from the Netherlands identified several frequency maps in people with tinnitus and clinically normal hearing compared to matched controls (Figure 2) [4]. Yet these maps did not significantly differ between the two groups. It is possible that large-scale reorganisation is more a substrate of hearing loss than tinnitus. Further research is needed to address this question.
Figure 2: An example of the orderly frequency gradient around human primary auditory cortex in response to tones presented at different frequencies. The colour scale proceeds from blue (low frequencies) to red (high frequencies) and the view shows the left hemisphere.
Neural synchrony in auditory cortex
From the animal model, hyperactivity in spontaneous firing, bursting discharges and increased cortical or brainstem / thalamic neural synchrony have all been put forward as potential neurophysiological substrates for tinnitus; either alone or in combination [5]. Of these three types of neural activity, changes in neural synchrony measured in animal models of hearing loss appear to correspond most closely to the frequency profile of tinnitus and hearing loss measured in human tinnitus patients [5].
The EEG signal measured at the scalp depends not only on the amplitude of underlying neural activity but also its spatial synchronisation and so it is a good biomarker in this case. Magnetencephalography (MEG) is a companion method that measures the magnetic equivalent of the electrical brain activity. A role for synchronous activity in tinnitus is implicated by a report of altered spontaneous oscillatory MEG brain activity in a group of people with tinnitus compared with normal hearing controls with no tinnitus [6]; characterised by a reduction of the alpha rhythm (~10 Hz) and an increase of the delta rhythm (< 4 Hz), particularly in the vicinity of bilateral auditory cortex. In a further report from the same group, gamma rhythms (50–60 Hz), examined during brief periods of marked enhancement of slow-wave activity, were greater in people with tinnitus than in normal hearing controls with no tinnitus [7]. Not only was this effect generated in bilateral auditory cortices, it also tracked the laterality of the tinnitus percept. While alpha activity may be a useful proxy for estimating the integrity of the excitatory-inhibitory neuronal balance, yet again it is not possible to clearly separate those large-scale reorganisations due to hearing loss from those due to tinnitus. Further research is warranted.
Structural differences in the vicinity of primary auditory cortex
An underlying assumption made by workers in the field is that if functional (i.e. perceptual or behavioural) changes occur, then there are likely to also be changes in the properties of the neural circuitry or the anatomical organisation mediating that function. The nature of those abnormalities can shed light upon the pathophysiological mechanisms that generate and maintain tinnitus in the brain. Voxel-based morphometry is a magnetic resonance imaging (MRI) technique that deals with differences in the local composition of brain tissue (i.e. grey and white matter).
Several studies have used voxel-based morphometry to disentangle effects related to hearing loss and tinnitus by comparing three groups: i) people with tinnitus and hearing loss, ii) people with hearing loss and no tinnitus, and iii) normal-hearing controls with no tinnitus [8,9]. Findings are somewhat mixed. For the USA team [8], the major finding was both grey and white matter changes in the vicinity of the auditory cortex for group ii) relative to the other two groups suggesting that sensory deprivation may be the primary driver for long-term structural changes. For the Netherlands team, a spatially restricted analysis revealed a grey matter increase in the left primary auditory cortex of the tinnitus patients compared to the other two groups, indicating that tinnitus could be a driver for significant structural change [9]. These (and other) contradictory findings are most likely due to different analysis methods employed, the relatively small sample sizes in most studies, and / or the heterogeneity of the experimental sample. Cooperation across different teams to replicate recruitment criteria, data acquisition and analysis strategy is needed to address this question.
Concluding remarks
While damage to the cochlea can produce anatomical, physiological and biochemical changes in peripheral and central auditory pathways, not all individuals with noise-induced hearing loss develop tinnitus. The neural mechanisms underlying tinnitus expression are not completely known. While human brain imaging has the potential to shed light on this puzzling dilemma, the field lacks carefully controlled studies that separate out the major confounding variable of hearing loss.
References
1. Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Sp Lang Hear Res 2005;48:1204-35.
2. Rajan R, Irvine DRF. Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory organ damage. Audiol Neuro-Otol 1998;3:123-44.
3. Wienbruch C, Paul I, Weisz N, Elbert T, Roberts LE. Frequency organization of the 40-Hz auditory steady-state response in normal hearing and in tinnitus. NeuroImage 2006;33:180-94.
4. Langers DRM, de Kleine E, van Dijk P. Tinnitus does not require macroscopic tonotopic map reorganization. Front Syst Neurosci 2012;6:2.
5. Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing Ears: the neuroscience of tinnitus. J Neurosci 2010;30(45):14972-9.
6. Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med 2005:2:e153.
7. Weisz N, Müller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J Neurosci 2007;27:1479-84.
8. Husain FT, Medina RE, Davis CW, Szymko-Bennett Y, Simonyan K, Pajor NM, Horwitz B. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res 2011;1369:74-88.
9. Boyen K, Langers DR, de Kleine E, van Dijk P. Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear Res 2013;295:67-78.
Further reading
A special issue of Hearing Research (Elsevier) will be dedicated to the topic of Human Auditory Neuroimaging, with invited reviews from experts including Husain, Langers and Weisz; and guest edited by Hall and Langers. It is expected to be published in January 2014,
http://www.journals.elsevier.com/hearing-research/
Declaration of Competing Interests
Prof Hall is funded by the National Institute for Health Research (NIHR). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health. The author has made all reasonable efforts to ensure that the information provided in this review is based on available scientific evidence.