Auditory processing disorder (APD) has had a controversial history, stemming mainly from lack of scientific rigor and accepted clinical definition. That situation is now changing. Driven by the huge number of people with unaddressed listening difficulties, basic discoveries in neuroscience, and translational research investment, we are starting to unravel the separate roles of the ear, the auditory nervous system, and the cognitive (‘thinking’) ability of the human brain, as David Moore explains.
Auditory processing may be defined as the coding and recoding by the central auditory nervous system (CANS) of acoustic signals received, shaped and transduced by the ear. Because sensitivity and frequency specificity of hearing are largely determined by the ear, it is assumed that the pure tone audiogram accurately reflects that sensitivity and specificity. When people complain of hearing difficulties, the audiogram has thus become the gold standard measure of ear function.
However, it has long been recognised that some people with audiometrically ‘normal’ hearing still have hearing difficulties, and that the audiogram is not a very good predictor of meaningful, attentive hearing (listening) at sound levels above threshold. For people with a normal audiogram, it has therefore been assumed that the explanation of any hearing difficulties must lie beyond the cochlea, in the CANS. The concept of ‘auditory processing disorder’ (APD) began to be developed by audiologists some 50-60 years ago in an attempt to describe these difficulties.
What’s new?
There is a growing realisation that both normal hearing and all forms of hearing difficulty are multifaceted. Hearing necessarily involves the ear, the CANS, and other brain systems, including attention, memory and vision. For language, that most human of functions that normally begins with high quality speech perception, recoding of the pre-linguistic auditory signal appears to lie mainly outside the conventional CANS, beginning in the superior temporal sulcus [1], a brain area just lateral to the auditory cortex. At the other end of the system, in the cochlea, we see growing evidence for ‘subclinical’ or ‘hidden’ hearing loss that occurs despite normal audiometry. For example, noise damage and normal ageing are both associated with a partial loss of synapses between the inner hair cells and the auditory nerve, at least in animal models [2]. This results in reduced output of the cochlea at higher sound levels. Other examples of hidden hearing loss in humans include subclinical OAE deficits, very high frequency (>8 kHz) audiometric insensitivity, and a relation between poor speech-in-noise hearing and desynchronised brainstem frequency following responses among normally hearing young adults.
“I know of no clear evidence for an involvement of the CANS in APD that cannot be explained either by a conventional hearing loss or by a frank neurological lesion.”
These recent discoveries in neuroscience have several implications for APD, and for audiology in general. First, any test of hearing, but particularly a speech-based test, necessarily involves a great deal of brain processing beyond the CANS. It is therefore inappropriate to assume that the CANS is the site of problems underlying APD. In fact, I know of no clear evidence for an involvement of the CANS in APD that cannot be explained either by a conventional hearing loss or by a frank neurological lesion. Second, a normal audiogram does not imply that any remaining hearing difficulty is a dysfunction of the nervous system. It may be due to a hidden hearing loss restricted to or originating in the cochlea. Removing the prefix ‘central’ from APD, as in CAPD or (C)APD, would be a symbolic recognition that we should not rule out a possible role of the ear in APD. Third, because of the involvement of ‘higher level’ or ‘cognitive’ function in hearing, it is necessary to educate the profession about these aspects of hearing.
All professional societies recommend the use of a multi-disciplinary approach to APD. While this approach is commendable in many respects, the audiologist needs to be familiar with at least some basics of cognitive science to understand and coordinate care.
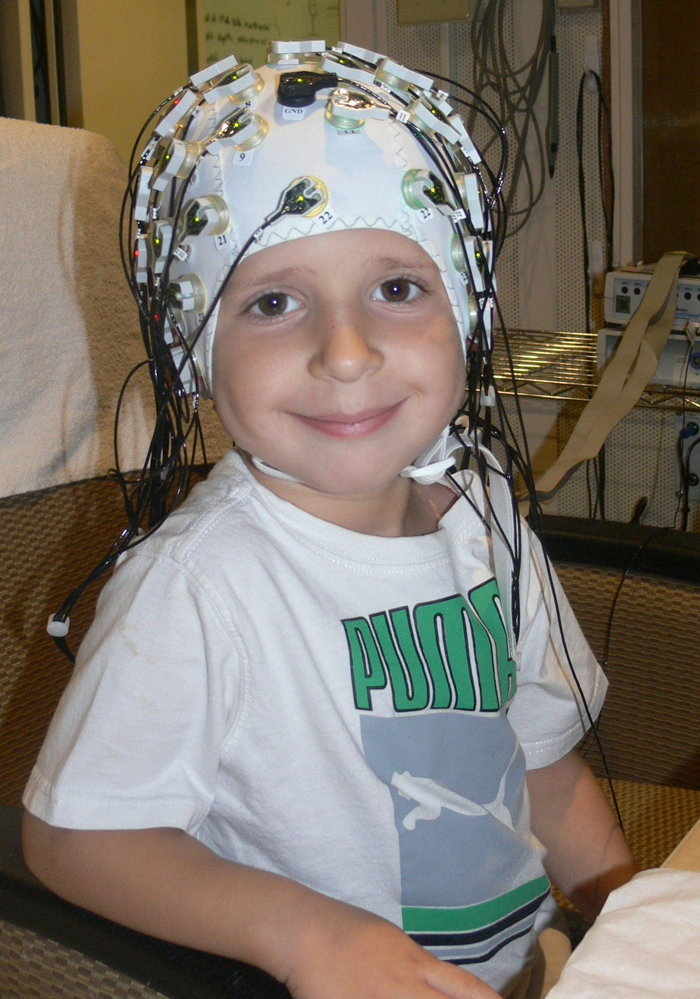
Cutting edge approaches, such as high density EEG recordings, are helping us parse the role of bottom-up and top-down processes in auditory processing. (Photo: Andrew Dimitrijevic)
Another recent development has been the growing recognition that the clinical history of a child or adult presenting with unexplained hearing difficulties should play a more formal role in the diagnosis of that individual. This may sound like an obvious statement, but the clinical ‘handling’ of a child suspected as having APD suggests otherwise. Typically, they have a detailed clinical history taken, as well as a detailed audiological assessment. They are then given a battery of tests that purports to test for APD. The final decision as to whether the child has an APD is strongly influenced by the results of that battery [3], in addition to the clinical history. However, there is little or no rigor applied to the structure of the history or to appropriate interpretation of the responses.
For that reason, the British Society of Audiology Special Interest Group on APD recommended an early and central role in evaluation for a well designed, reliable and valid questionnaire [4]. Such a questionnaire could additionally act as a screening instrument and as an outcome measure for interventions.
APD becomes respectable
In October 2014, Erick Gallun and KC Lee organised a session at the Acoustical Society of America meeting entitled ‘Physiological and Psychological Aspects of Central Auditory Dysfunction’. Several of the presentations in that session may have been almost unrecognisable to a practising clinician faced with a child who has unexplained learning and listening problems, but they did serve two important purposes. First, they represented the considerable range of scientific methods and observations that are now being applied to APD. For example, two presentations considered the effects of traumatic brain injury resulting from blast damage in veterans of the Gulf wars. Evidence was presented that many such cases, although not involving threshold insensitivity or clear neurological lesions, nevertheless resulted in impaired performance on clinical APD tests. Other presentations focused on binaural processing and working memory, and on perceptual learning as a model for APD intervention. The second point is that the mere existence of such a session at the meeting of a society that has, perhaps reasonably, shunned APD for so many years is evidence of a ‘coming of age’ of APD as a science.
What does this mean for the clinician?
The majority of audiological services worldwide do not offer an APD assessment. They typically argue that we do not know what APD is, how to assess it, or how to remediate it. As researchers in this field, our aim is to address each of these issues. I have tried to outline above a current view of what APD is and, in particular, what biological mechanisms might underlie it. Further information and views can be found in the scientific literature – see especially papers by Harvey Dillon and colleagues, and David DeBonis [5]. To assess APD, I believe the first step is a questionnaire, as above. Following initial audiological evaluation, hierarchical assessment using a limited number of tests has been recommended and tested clinically with children in Australia [6]. Limited but robust and well-normalised testing recommended by DeBonis [5] consists of two questionnaires (one on executive function and one on communication skills) and two speech-in-noise tests (WIN and BKB Sentences). These two approaches are based on published, peer-reviewed evidence and can help guide clinicians to a better assessment framework.
“These two approaches are based on published, peer-reviewed evidence and can help guide clinicians to a better assessment framework.”
Of the many remediation options presently available, only a few seem to have any degree of scientific support. These include environmental and behavioural modification, such as improving room acoustics (e.g. in classrooms), looking at a speaker, and the use of remote microphone wireless devices. Other approaches are the use of stimulant medication, shown to improve auditory processing, and some forms of auditory training. Regarding training, the literature remains divided, with some studies finding in favour, but the majority against the necessary ‘far transfer’ of specific skill training [7]. Music training, learning a second language, and physical exercise have all been advocated, with some evidence for improving cognitive skills underlying listening.
Clearly, there is much to be done in a field that is only beginning to offer a serious scientific basis for clinical application. Let us take stock, however, of how many people could benefit from this work. Several recent estimates put this number at the 1-5% of audiology patients who have normal audiograms. But to this relatively small number could be added the many older people who have listening difficulties, ‘secondary’ APD [4], additional to an audiometric hearing loss. Finally, there are the 10% or so of all children who often report listening difficulties but have other, more well-recognised developmental learning disabilities. Together, these populations represent a major, but potentially treatable problem.
SUMMARY
-
Hearing necessarily involves the ear, the CANS, and higher brain systems
-
Subclinical pathology of the ear or language, attention and memory problems may contribute to APD
-
Questionnaires and speech-in-noise tests have been recommended for APD assessment
-
Effective remediation may include environmental modification, remote microphone devices, and some brain training exercises.
References
1. Overath T, McDermott JH, Zarate JM, Poeppel D. The cortical analysis of speech-specific temporal structure revealed by responses to sound quilts. Nat Neurosci 2015;18(6):903-11.
2. Liberman MC. Hidden hearing loss. Sci Am 2015;313(2):48-53.
3. Emanuel DC, Ficca KN, Korczak P. Survey of the diagnosis and management of auditory processing disorder. Am J Audiol 2011;20(1):48-60.
4. Moore DR, Rosen S, Bamiou D-E, et al. Evolving concepts of developmental auditory processing disorder (APD): a British Society of Audiology APD special interest group ‘white paper’. Int J Audiol 2013;52(1):3-13.
5. DeBonis DA. It is time to rethink central auditory processing disorder protocols for school-aged children. Am J Audiol 2015;24(2):124-36.
6. Cameron S, Glyde H, Dillon H, et al. Results from a national central auditory processing disorder service: A “real world” assessment of diagnostic practices and remediation for CAPD. Seminars in Hearing 2015;36:216-36.
7. Makin S. Brain training: Memory games. Nature 2016;531(7592):S10-11.
Declaration of Competing Interests: David Moore declares that he is co-inventor of the ECLiPS questionnaire, used to assess listening difficulties in children. The ECLiPS is licensed by MRC Technology, London.
ABOUT THE AUTHOR
Dave Moore studies hearing in children. His primary focus is on children with listening difficulties. The research aims to identify processing problems in the ear and brain that are not detected in a standard evaluation. Additional current interests include very early development of hearing (from ages 1-4 years) and neurogenetics. In 2015 he received the Career Award in Hearing or Balance of the American Academy of Audiology. In 2016 he delivered the TS Littler Lecture to the British Society of Audiology.




