“The more you know, the harder it is to take decisive action. Once you become informed, you start seeing complexities and shades of gray. You realize that nothing is as clear as it first appears. Ultimately, knowledge is paralyzing.”
Calvin, from the cartoon strip Calvin and Hobbs, may not have been referring to the investigation of thyroid nodules, but for many radiologists dealing with the almost daily ‘incidental thyroid nodule’, the author Bill Watterson was pretty close to the mark.
As a radiologist with a particular interest in thyroid nodules, I have seen a significant improvement in our ability to image nodules with newer and higher resolution ultrasound machines – and yes, it is about complexities and shades of grey. It is not, however, paralysing. A burgeoning volume of evidence in the medical literature supports our ability to assess the likelihood of malignancy based upon nodule features. Contrary to the above, I will look at a number of aspects of thyroid nodule imaging and provide an up-to-date assessment based upon our radiology literature.
The incidence of thyroid cancer is increasing
True. The incidence of thyroid cancer has increased significantly over the last 30 years, and it is now the most common endocrine malignancy. In Europe, rates have increased by 135% overall, with a 159% increase amongst women [1]. Drilling deeper into the statistics reveals some interesting facts. Various studies have concluded that the increased incidence is due to two factors: firstly, better quality imaging modalities, detecting ever more increasing numbers of thyroid nodules which undergo cytological evaluation; secondly, improved cytological and pathological techniques, detecting smaller micro-carcinomas which previously would not have been identified.
The argument for imaging being a causative factor is further supported by the current situation in North America, whereby more thyroid cancer diagnoses are made from nodules discovered incidentally on other imaging modalities, than from palpable or clinically detectable nodules. The increased usage of imaging (CT, MR, ultrasound) has been replicated in all major healthcare nations, and incidental thyroid nodules have become the bane of many a radiologist, ENT surgeon and endocrinologist.
An important point needs to be made here: when I say bane, I refer to ‘a source of persistent annoyance or exasperation’, and not the alternative definition, ‘something that causes death or destruction’. The incidence of thyroid cancer has risen sharply, but with stable, static mortality rates over the last 30 years. The majority of new thyroid cancer diagnoses are almost exclusively small papillary cancers <15mm diameter, whose malignant potential is questionable in patients with no history of prior irradiation, or positive family history of thyroid cancer. These findings, and many others are covered within the British Medical Journal (BMJ) article by Brito et al. [2], as part of a series of articles regarding over-diagnosis of pathology. Whilst there are some valid questions about this article in the online, the authors should be applauded in raising the issue of over-investigation of thyroid nodules.
Ultrasound is rarely helpful in assessment of thyroid nodules
False. Contrary to the last British Thyroid Association & Royal College of Physicians (BTA / RCP) Guidelines for the Management of Thyroid Cancer (2007) [3], there is a wealth of medical literature regarding the ability of ultrasound (US) to detect thyroid cancer. As the Royal College of Radiology representative on the latest BTA / RCP Guidelines Group for Management of Thyroid Cancer, I have become intimately acquainted with many of these articles.
We know that no single US sign has high enough sensitivity or specificity to confirm or exclude malignancy. What is becoming apparent however is that a combination of signs or US features can be used in summation to provide an excellent guide to the risk of underlying cancer. Multiple papers confirm the ability of US to ‘risk stratify’ nodules, and provide an accurate assessment, firstly of whether a fine needle aspiration (FNA) is required, and secondly which nodule should be sampled. Further technology such as US elastography, assessing how firm or compressible a nodule is, are still being assessed.
A number of consensus documents or guidelines are available to suggest when we should FNA thyroid nodules. A study by Ahn et al. [4] compares the use of three such sets of guidelines, and their ability to detect thyroid cancer. If we look more closely at this paper, we have the Kim Critera [5], the American Association of Endocrinologists (AACE) [6] and finally the Society of Radiologists in Ultrasound (SRUS) [7]. The study involves 1395 nodules with imaging, cytology and pathology for correlation. The Kim Criteria suggest FNA of any nodule demonstrating one of four features: marked hypoechogenicity, irregular margins, microcalcification, and a ‘taller-than-wide’ shape (i.e. AP>TR when measured in the axial or transverse plane). AACE use the same four criteria, but require marked hypoechogenicity plus one other feature from the remaining three. The SRUS guidelines are more complex, and suggest FNA of nodules >10mm with microcalcification, >15mm if solid, >15mm with coarse calcification, and >20mm if solid and cystic.
The results of the paper are interesting as regards the detection of thyroid cancer: the Kim Criteria produce sensitivity of 92.7%, specificity of 80.9% and negative predictive value (NPV) of 97.3%. The AACE Criteria produce sensitivity of 74%, specificity of 94% and NPV of 95%. The SRUS Criteria for FNA (taking into account nodule size) produce sensitivity of 35%, specificity of 54% and NPV 80%.
There are a few important points to consider: it is papillary cancer that is accounting for the increase in thyroid cancer incidence, and the four US features used in the Kim and AACE criteria are the cardinal features of papillary cancer (Figures 1-4). For high sensitivity, the Kim Criteria should be used, for high specificity, AACE criteria. The use of nodule size reduces sensitivity and specificity quite markedly.
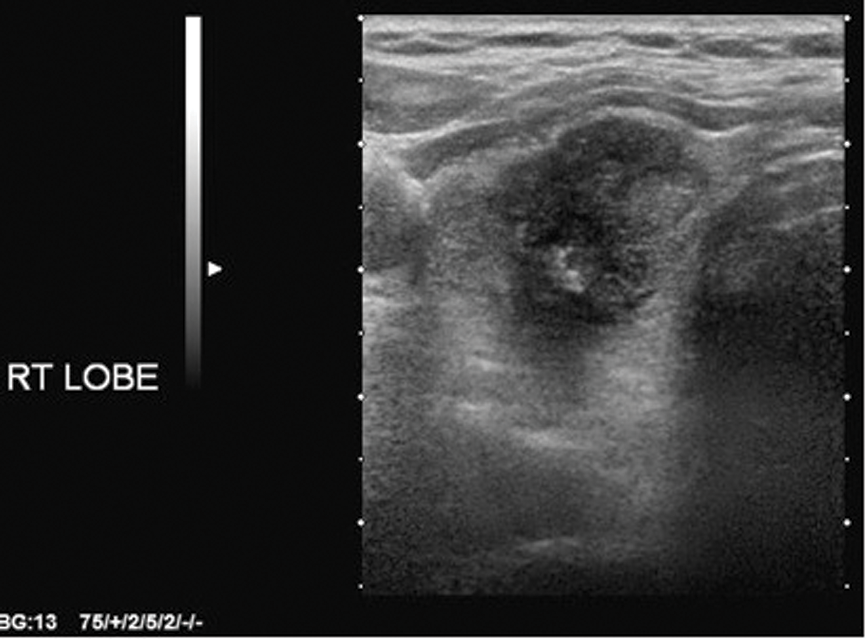
Figure 1: Solid, hypoechoic right lobe of thyroid mass.
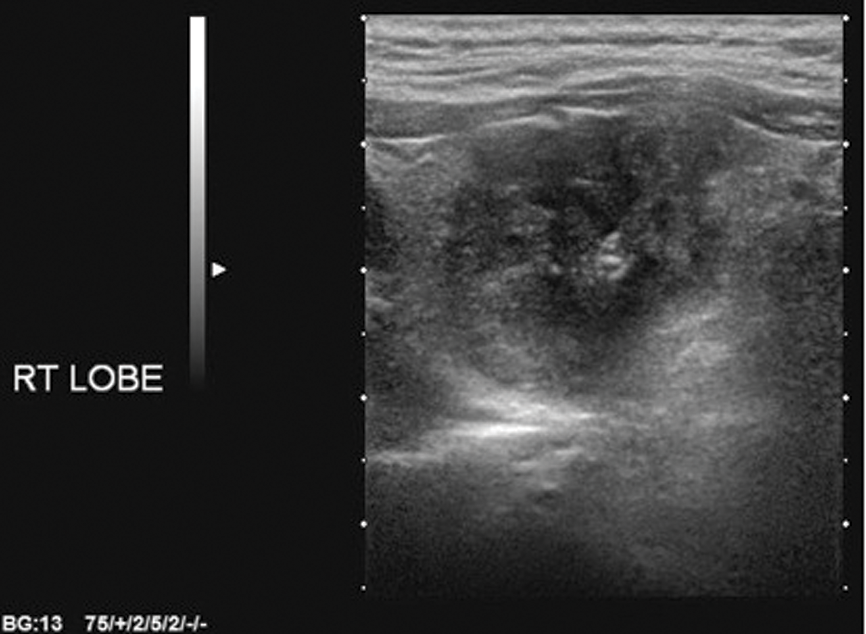
Figure 2: Ill-defined margins.
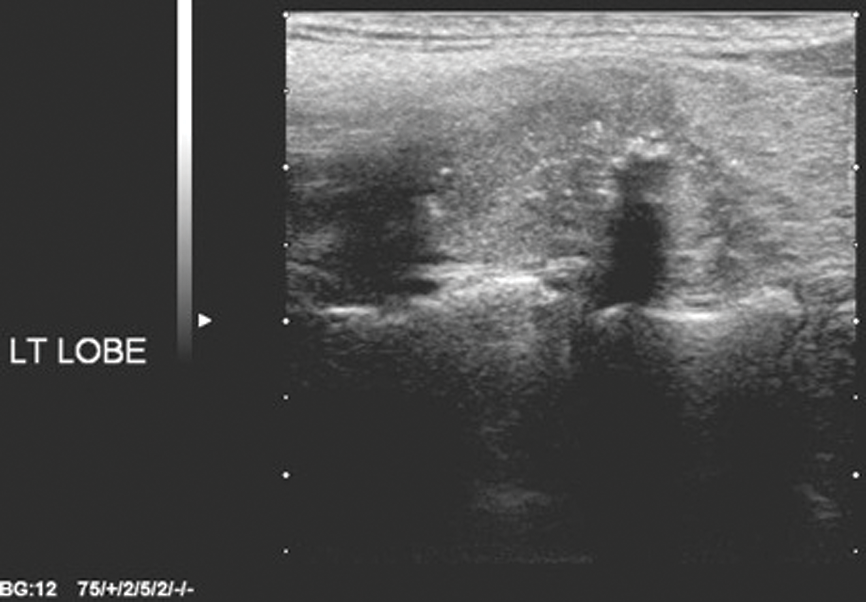
Figure 3: Microcalcification.
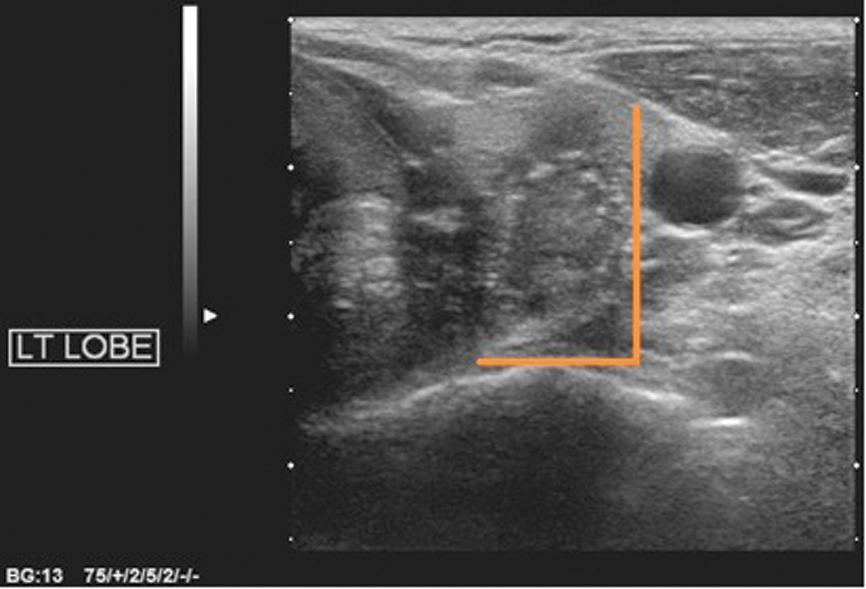
Figure 4: Taller-than-wide (AP>TR in transverse plane).
In addition to the Kim and AACE Criteria, the excellent Mayo Clinic Thyroid US Chart (Practical Approach to Thyroid Nodules) provides a straightforward guide to thyroid nodule management. Nodules are classified as ‘Almost Certainly Benign’, ‘Indeterminate’ or ‘Worrisome for Malignant’. Benign nodules do not undergo FNA. Indeterminate nodules undergo FNA if there are associated risk factors in the clinical history, and all worrisome nodules undergo FNA. Scoring systems such as this have been shown to improve patient management by improving cost-effectiveness and reducing unnecessary FNA. Scoring systems also allow effective training, are reproducible with low inter-observer variability, and allow effective audit and follow-up.
FNA of a dominant nodule in a multinodular goitre should be performed
False. This is a common but mistaken practice. Nodule size is not an indicator of malignant potential. Numerous studies now confirm that US is still reliable in nodules >3 or 4cm, to assess the malignant potential and guide FNA. Previous studies suggesting a malignancy rate of >20% in nodules >4cm have small sample sizes, and were performed on older US machines. It seems counter-intuitive to consider slow growing nodules, present for years, as suspicious based purely on a size measurement. Furthermore, this practice tends to encourage lazy radiology, opting to FNA the largest nodule rather than assess all the nodules and base our decision to sample upon US features associated with malignancy.
Benign Thy2 FNA result needs repeating within six months:
False. FNA results need correlation with the clinical picture and the ultrasound appearances. Repeat FNA of all nodules that have yielded a Thy2 result is not cost-effective, and increases patient anxiety. Kwak et al. [8] compare rates of malignancy within nodules based upon the initial US appearances, in more than 1300 nodules with an initially benign FNA result. The study shows that nodules with benign US appearances and a Thy2 FNA have extremely low rates of malignancy (99.4% benign). This does not warrant repeat sampling. A nodule with suspicious US appearances but initial Thy2 FNA does require repeat sampling, as malignancy rates are significant, in excess of 20%.
Nodule growth is suspicious for malignancy
False. Following an US and FNA, there is disagreement in the next best step. Follow-up US has been used in many centres, but the presence or absence of nodule growth does not predict either benignity or malignancy. It is again the nodule appearance on US, which is the best predictor. Interval growth has a poor positive predictive value (PPV) for malignancy, and studies now show that follow-up US increases costs and unnecessary FNA, without an increase in diagnosis of malignancy [9]. Another study looking at the natural history of nodules with prior benign FNA [10] showed that 294 of 330 nodules grew during a five-year follow-up, all by >15% volume. An average volume increase of 69% was present in 74 nodules that underwent re-FNA, but only one of these showed thyroid cancer. Growth of nodules is an expected finding in benign nodules, and does not predict malignant potential.
Summary
Modern imaging has led to a huge tidal wave of incidental nodules and an increase in diagnosis of incidental, potentially indolent, thyroid cancers. Modern US machines are extremely effective in assessing nodules, and there is ample evidence to support the use of scoring systems and risk stratification for selecting which nodules, if any, to FNA.
Radiologists need to be proactive and encompass such scoring or stratification systems to improve patient management. Such pathways reduce unnecessary FNA, improve follow-up, and allow effective teaching, audit and assessment of our performance.
References
1. Cancer Research UK. Thyroid cancer incidence statistics.
www.cancerresearchuk.org/
cancer-info/cancerstats/types/thyroid/incidence
Last accessed March 2014.
2. Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ 2013;27:347.
3. British Thyroid Association and Royal College of Physicians. Guidelines for the management of thyroid cancer. 2nd edition. London, UK; British Thyroid Association and Royal College of Physicians; 2007.
4. Ahn SS, Kim EK, Kang DR, et al. Biopsy of Thyroid nodules: comparison of three sets of guidelines. Am J Roentgenol 2010;194:31–7.
5. Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine needle aspiration of nonpalpable solid nodules of the thyroid. Am J Roentgenol 2002;178:678-91.
6. Gharib H, Papini E, Valcavi R, et al. American Association of Clinical Endocrinologists and Associazone Medici Endocrinologi medical guidelines for the diagnosis and management of thryoid nodules. Endocr Pract 2006;12:63-102.
7. Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005;237:
794-800.
8. Kwak JY, Koo H, Youk JH, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology 2010;254(1):292-300.
9. Lee S, Skelton TS, Zheng F, et al. The biopsy-proven benign thyroid nodule: is long-term follow-up necessary? J Am Coll Surg 2013;217(1): 81-88.
10. Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Int Medicine 2003;138:315-8.
Declaration of Competing Interests: None declared.




