
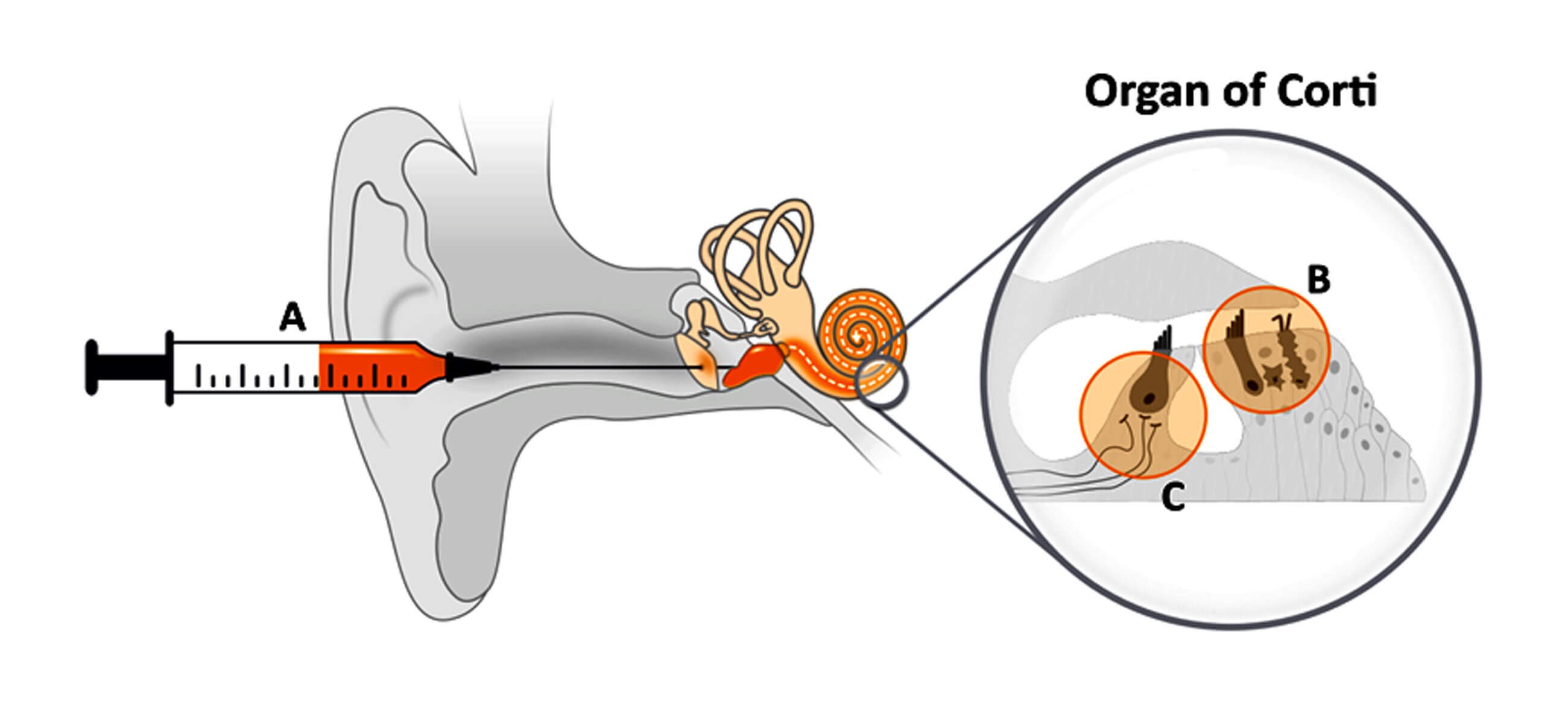
While corticosteroids are widely used to treat Sudden Sensorineural Hearing Loss (SSNHL), there is still no therapy with proven efficacy and regulatory approval for this debilitating condition. To address this high unmet medical need, AudioCure Pharma is developing the investigative medicine AC102, which is applied via single intratympanic injection (A) and outperformed corticosteroids in hearing loss models. In these models, AC102 almost completely restored noise-induced hearing loss across all frequencies tested by preventing apoptosis of sensory hair cells (B) and synaptic disconnection of inner hair cells to the auditory nerve (C).
A Phase 1 clinical trial confirmed the safety and tolerability of AC102 in healthy volunteers. The efficacy of AC102 is currently being investigated in a Phase 2 clinical trial in SSNHL patients. As this trial compares the efficacy, safety and tolerability of AC102 with that of corticosteroids, it ensures that all patients will be treated. The Data and Safety Monitoring Board (DSMB) reported no safety concerns after analysis of the first patient cohort.
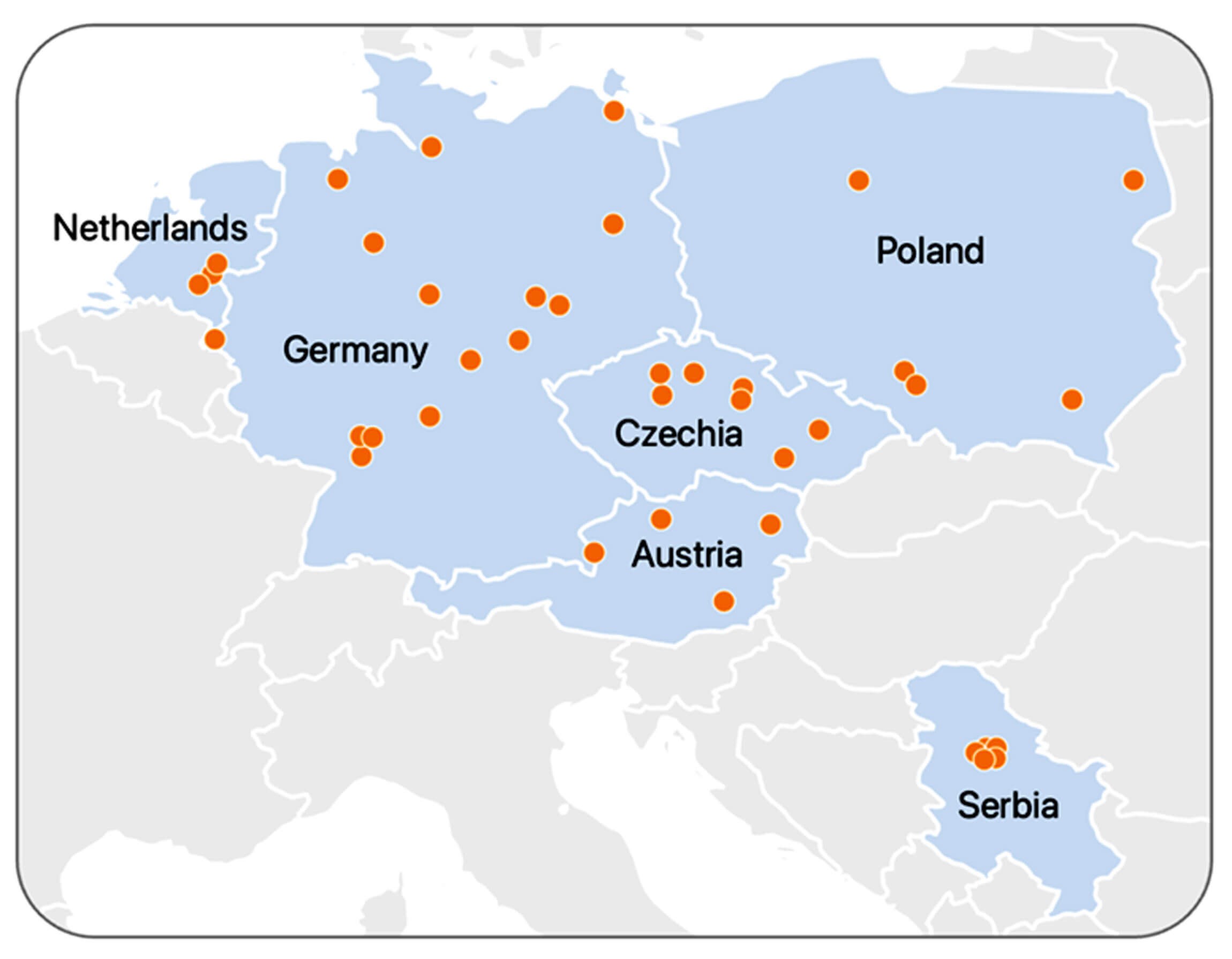
The study is conducted in up to 50 study sites across Europe including Germany, Austria, the Netherlands, Poland, the Czech Republic and Serbia. To be included in the study, patients must be aged between 18 and 85 and have a unilateral idiopathic hearing loss of more than 65 dB that has occurred no more than 120 hours previously. Patients with a recent history of hearing loss (one year), non-idiopathic hearing loss (e.g. otitis media, noise or tumor) or undergoing corticosteroid therapy cannot participate.
FURTHER INFORMATION:
To refer a patient to the AC102-201 trial, please find more information here and find your closest study site. If you have further questions, please contact trials@audiocure.com



