In the first of this two-part series, Martyn Barnes and colleagues discussed indications for functional endoscopic sinus surgery (FESS), the surgical objectives and techniques, patient expectations and the risks of surgery [1]. In this second and final part, the authors discuss how to make FESS easier, review the imaging pre and intra-operatively, optimise the operative field, decide the extent of surgery, and manage any complications.
Introduction
As we detailed in part one, we have written these manuscripts to highlight some of the most important things we feel we have learned in our practice of functional endoscopic sinus surgery as it has evolved over the last few decades. No short article can in itself be a substitute for experience, but we hope it provides some inspiration to our juniors, and some consolidation and reinforcement to our trainees and peers. We would welcome further dialogue to add to our thoughts - see author contact.
Imaging review
Preoperative sinonasal CT imaging is essential to FESS surgery. Ideally the CT data should be provided in thin-slices (less than 1mm thick) including the full volume of the paranasal sinuses. The images should be available during surgery in a 3 axis viewer, preferably one with the facility for multiplanar reconstruction (MPR).
In our theatres, most of us use the Osirix MD viewer, installed on a Mac laptop covered with a sterile drape (through which the touchpad can be used). This allows us to review the anatomy whenever we feel uncomfortable about our surgical position without de-scrubbing, or using an assistant. Without this convenience, such checks are easily skipped and safety is compromised. We use this set up during most revision, frontal sinus, tumour, or ‘skullbase’ cases.
“In complicated cases or revision surgery, image guidance should be considered to allow safe dissection in an operative field with minimal or no landmarks.”
Before the case, it is helpful to perform an MPR to align the axial slices with the palate and skull-base, while correcting any angulation of the coronal images to obtain symmetric views. Window-levels are optimised for bone definition, rotation corrected for each view, and the views maximised by zoom. We then study the scans from three different perspectives as follows.
Operative CT - sinonasal anatomy
Studying and understanding an individual patient’s radiological anatomy will facilitate a safe and effective dissection. It is usually easiest to identify deviations from ‘normal anatomy’, recognising features such as a concha bullosa, Onodi, and Haller cells to allow operative orientation and maintain safety. The complex anatomy of the frontal recess is defined by the relationships of the basal lamellae of the uncinate process and ethmoidal bulla and their insertions into the lamina papyracea and skullbase. Understanding this anatomy, then demonstrating the same during surgery by probing will then facilitate a complete, safe, and mucosa preserving technique.
Operative CT - the disease
Most FESS is performed for chronic rhinosinusitis. The scan should have been studied well before surgery, but immediate preoperative review is a final opportunity to recognise a sinonasal mass and avoid the unexpected complications of operating on a vascular tumour or meningo / encephalocoele. Findings of an antrochoanal or frontochoanal polyp must be recognised; dictating a surgical approach to allow complete clearance. Bony changes in a unilateral polyp may indicate an inverted papilloma or neoplasia, necessitating histopathologic analysis.
Operative CT - risks for surgery
Many advocate the CLOSE mnemonic - a reminder to recognise risks by their anatomic location:
Cribriform plate - defects, deep (vulnerable) lateral lamellae, or disorientating asymmetry.
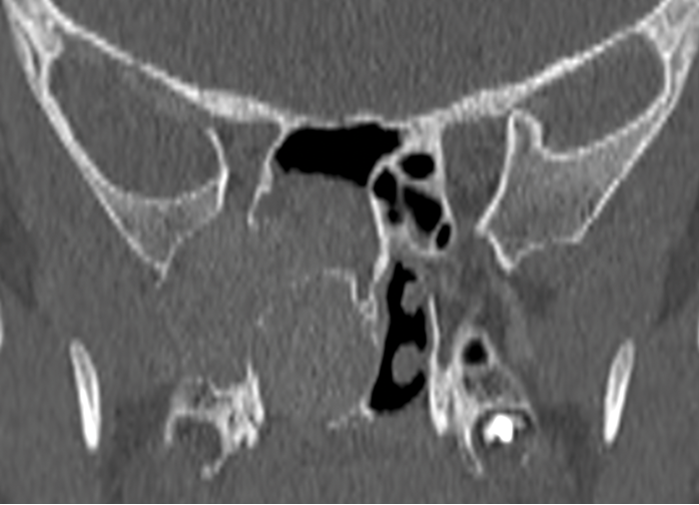
Lamina papyracea - defects (from prior surgery or injury) or a low uncinate orbital insertion.
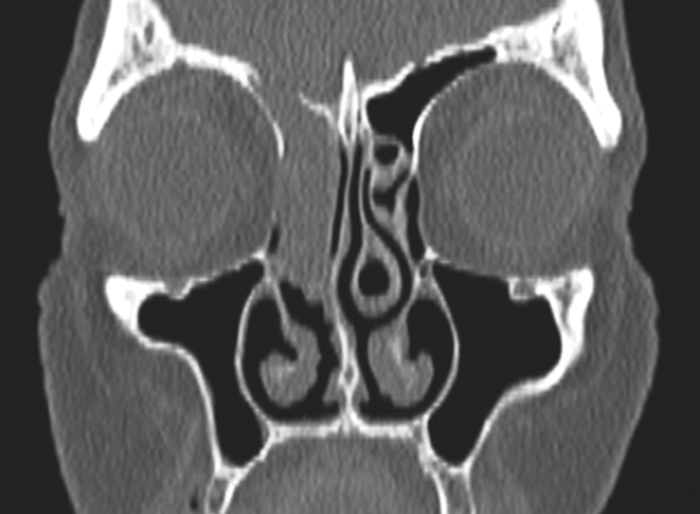
Onodi cells - most easily seen on coronal views, especially after a well performed MPR.
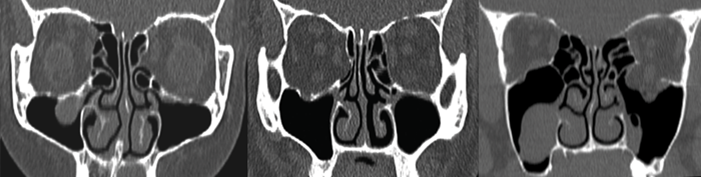
Sphenoid relations - especially an optic nerve or carotid within a bony or dehiscent mesentery.
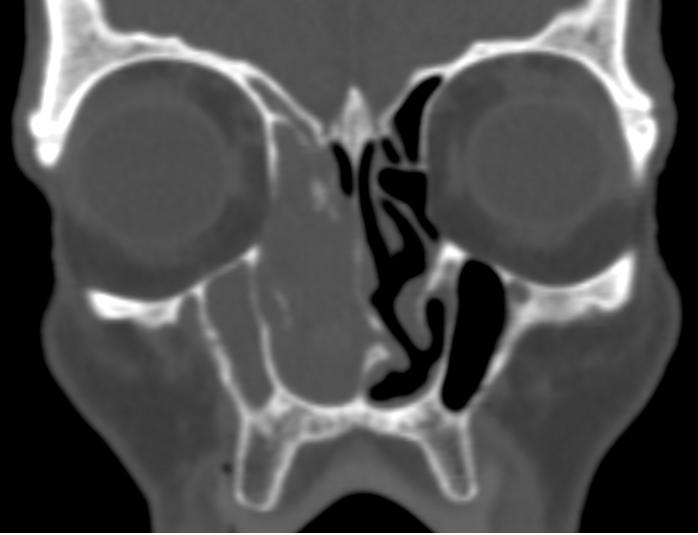
Ethmoid artery - noting how far beneath the skullbase the anterior ethmoid artery lies.
In complicated cases or revision surgery, image guidance should be considered to allow safe dissection in an operative field with minimal or no landmarks. Image guidance is now available in most large centres and should be considered the standard of care in complicated / revision cases.
Juvenile Nasal Angiofiboma.
Meningoencephalocoele.
Endonasal Malignancy.
Lamina Papyracea Defects.
Extent of surgery
The extent of FESS surgery performed is extremely variable, and for good reason. The authors generally perform more extensive or complete surgery than others, but we recognise arguments for and against conservative and extensive surgery. Few of these arguments are supported by good evidence, although many have face validity. Recent evidence has shown better long-term outcomes with early surgical intervention for sinus disease [2]. By extrapolation, one might expect better long-term outcomes with an extensive approach at primary surgery.
Arguments for conservative surgery
- Reduced operative time
- Reduced operative risks
- Reduced operator skill and experience required Arguments for extensive surgery
- Improved symptom resolution
- Improved duration of disease control
- Reduced recurrence rates
- Reduced operative time, risks, and difficulty for revision surgery
- Better training opportunities and surgical skill maintenance, especially for endonasal access in skullbase, tumour, and orbital procedures
The only solid recommendations we would make is that no surgeon should perform beyond their competency, and that sub-specialisation will drive forward standards in these cases. The frontal recess in particular should not be compromised by inexperienced or hurried surgeons. The sphenoid is much easier to approach, but carries the risks associated with carotid artery and optic nerve proximity.
Ultimately, the extent of surgery is best planned in consultation with the patient preoperatively, but determined at the time of surgery based on the operative findings - disease extent, favourable or unfavourable anatomy, and sometimes also the surgical field achieved on the day in question.
Making FESS easier
Once a surgeon has acquired some basic endoscopic surgical skills, procedures such as nasal polypectomy and basic maxillary FESS are usually routine - almost regardless of the setting. For more advanced procedures, a much greater preparation and attention to detail is required. The most challenging FESS cases tend to be frontal dissections. Given the tight confines, angled approach, and extremely variable anatomy of the frontal recess, the otherwise simple task of removing septations and preserving the outermost mucosal lining is much more challenging, and some simple preparative steps can make an enormous difference.
“The extent of FESS surgery performed is extremely variable, and for good reason. The authors generally perform more extensive or complete surgery than others, but we recognise arguments for and against conservative and extensive surgery.”
Similarly, if an endonasal procedure seems difficult mid-surgery, take a step back and ask why. More often than not, you will find problems with either the surgical field, the visualisation, or the equipment that can be remedied. Sometimes the view seems clear enough to operate, but something about the fidelity does not give us all the information we need to make operative decisions and progress with surgery. Frequently, an inadequate view is only evident when something else compounds the issue - e.g. a little bleeding reduces the light, and then the problem is obvious.
Preoperative considerations
- Pre-treatment of acute infective or grossly inflammatory disease with antibiotics and / or systemic corticosteroids may improve the operative field.
- Recognise that certain patients may be more challenging - e.g. patients with poorly controlled hypertension or cardiac disease precluding significant hypotensive measures.
- Stop warfarin for five days preoperatively and check the INR immediately before surgery.
- Stop aspirin and clopidogrel for seven days before surgery. In patients with cardiac stents or a significant ischaemic history, we will operate without cessation of aspirin.
- Consult the cardiologist if there is any doubt about withholding medication perioperatively.
- Consider endovascular control before / during surgery or early operative ligation of feeding vessels in vascular lesions.
Anaesthetic aspects
No single anaesthetic approach seems to give invariable bloodless fields, but…
- Work with the same anaesthetist - especially one experienced in hypotensive anaesthesia.
- Total intravenous anaesthesia (TIVA) with propofol and remifentanil is advocated.
- Beta blockers or clonidine may be helpful if relative hypertension or tachycardia persist.
- Mean arterial blood pressure and heart rate should be close to 60mmHg and 60/min.
- Tranexamic acid (1g i.v. on induction) may reduce bleeding and improve the field.
- A laryngeal mask airway may reduce postoperative cough, straining, and bleeding.
- Throat packs are usually inserted to prevent pooling oropharyngeal blood and aspiration.
An experienced anaesthetist knows best how to optimise the surgical field. It is not wise to suggest anything they are unhappy with. No technique or numbers are necessarily wrong.
Preparing for surgery (positioning, nasal preparation, environment)
Your next challenge is to get the highest quality of views from the very start of the case.
Before we scrub, we position and prepare our patient:
- 15˚ of head elevation (reverse Trendelenburg) promotes venous decongestion.
- We use a Moffetts-like solution (4mL 1:1,000 adrenalin, 4mL of 4% Cocaine, 8mL saline, and 4mL of 8.4% sodium bicarbonate added last to avoid precipitation). This is applied on neurosurgical patties to the middle meatus, inferior turbinate and anterior nose / vestibule.
- Immediately before the first incisions, we infiltrate with bupivacaine and adrenalin - raising a small depot in the areas of the sphenopalatine foramen, the mucosa above the axilla of the middle turbinate, and the columella artery (depending on the procedure planned).
- Moist nostril hair can foul the endoscope on every pass. In long cases or hairy patients, we trim this before surgery with curved, rounded tip scissors or avulse with a needle-holder.
We always operate in a darkened room (some recommend green light) - even with the best of monitors, a much better view is achieved.
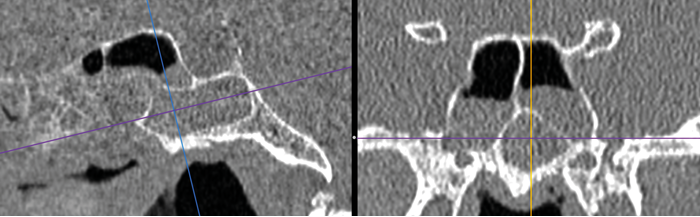
An Onodi Cell.
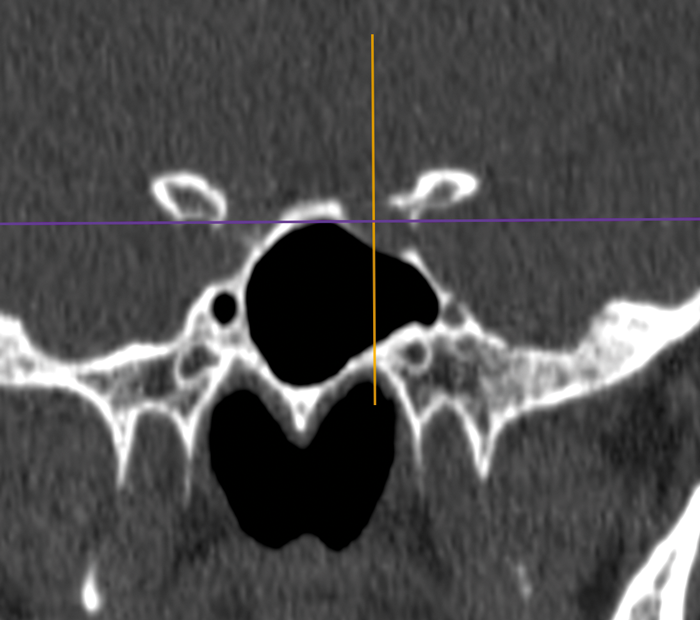
Sphenoid relations.
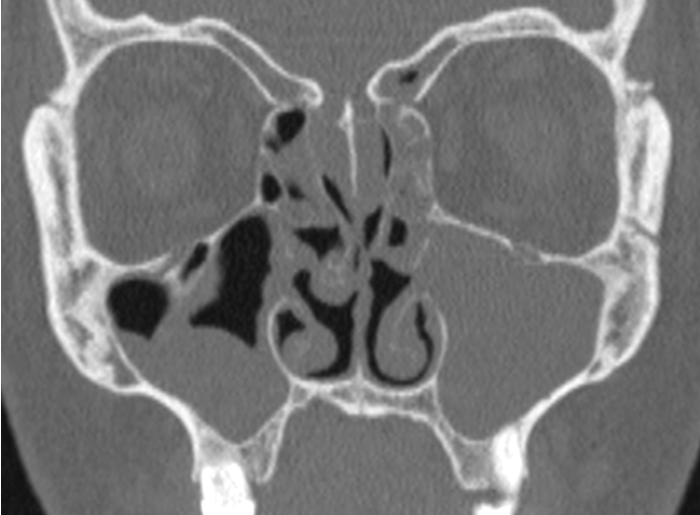
Anterior Ethmoid Artery.
Equipment checks
We encourage you to develop a critical eye - look twice at your equipment to ensure it is making your job as easy as possible. Check your camera, light source, cables, and scopes at the start of each case and try another if any component is found wanting. Ensure correct zoom, focal distance, white balance, and clean optics (endoscope and camera surfaces). Exclude broken scopes and optical fibres. Light cables and endoscopes can be checked by directing one end at a room light and ensuring all fibres at the distal end are lit.
You may be labelled as fussy, but involving your team in the process will help - let them recognise the problem, demonstrate to them the difference between a good and a bad view, and show them the improvement once the issue has been resolved. You will find that longer term solutions are found and your less fussy colleagues will benefit from well-maintained equipment too.
Some equipment problems are difficult to check for, so now have a look into the middle meatus. Fogging may resolve as the scope warms in use, but if it persists or you have any concerns about the view at all, I suggest you immediately change to one of your other scopes as a comparison
(I use a 0˚ primary scope and a 45˚ secondary). The secondary endoscope is often less used and in a better condition. If the view is much improved, request a replacement primary endoscope.
Endoscope handling
Your challenge now is to keep your view clear. We recommend the following measures:
- Hold your endoscope still in a clear part of the nasal cavity away from any bleeding sites.
- Stabilise your grip against the vault of the nose.
- Ensure your position (seated or standing) is a stable and comfortable one.
- Hold it away from the operative site, but close enough to maintain a good view.
- Position the tip so that gravity will not foul it (difficult while drilling the frontal beak).
Clearing your scope - work through these steps until the view is clear
- Rinse with the endoscrub, long enough for high flow mode if required (newer units only).
- Remove the endoscope from the nose and wipe (damp gauze) the sheath to the tip.
- Remove the sheath and wipe the endoscope shaft from light post to tip. Reinsert the shaft while rinsing through with the endoscrub.
- Partially remove the endoscope from the sheath and use an olive tipped suction to clean any fibrin / clot from inside the sheath.
- If a problem with the view persists, go back through your equipment checks.
Clearing your field (and taking a moment to rest your mind)
- Use atraumatic (olive tipped) suctions to clear any blood.
- Try not to suck directly on tissues.
- Suction can be augmented with wash from the endoscrub.
- Apply neuropatties soaked in adrenalin or Moffett’s solution. Suction the patties before removal.
- Wash the operative field - saline delivered quickly by 30ml syringe into the nasal vestibulum, or onto the surgical site / a sinus cavity through a curved suction. A good wash will clear the operative site, allow clean passage for the endoscope and avoid a dark field that reduces light.
- Change sides - a change is as good as a rest, and allows time for contralateral haemostasis. Always leave neuropatties in the nose while operating on the contralateral side.
Re-establishing orientation
When you have lost where you are in a FESS, look for well-maintained landmarks. Rarely is it impossible to identify the maxillary sinus ostium, lamina papyracea, orbital floor, choana, the root of the middle turbinate, nasal floor, or the skull base in the sphenoid or posterior ethmoid. Correlate everything you see with the scans in all three axes and, if available, use image guidance.
Controlling bleeding
It is better to prevent rather than control intraoperative bleeding, so you must be constantly aware of the location of the sphenopalatine artery, septal branch, anterior ethmoid and turbinate arteries (the ‘named arteries’). Work cautiously in areas of proximity or at times of uncertainty.
Rarely does any other bleeding fail to respond to conservative measures (washes, patties, time). If you are confident therefore that the named vessels have not been injured, control of bleeding will likely be achieved without diathermy.
Where necessary, bipolar diathermy is safest. Monopoly diathermy must only be used well away from the optic nerve. Thankfully, the region of the sphenopalatine artery is well away from the optic nerve and although best avoided, cautious use around the anterior ethmoid generally causes no harm (staying away from the lateral lamellar of the cribriform plate to avoid CSF leaks).
Management of complications
Epistaxis
Where a named vessel (see above) has been injured, this may only become apparent some days later. In the case of suspected sphenopalatine artery or septal branch bleeding, surgical exploration is recommended - the use of temporising measures (e.g. nasal packs) may prolong bleeding, increase risks, and delay recovery. Meticulous surgery, good anatomical knowledge and the use of diathermy or ligation when necessary will prevent most postoperative epistaxes.
Epistaxis in recovery
Most epistaxes occurring in recovery are related to extubation and the awakening of the patient. These events are messy and cause significant concern, especially in inexperienced environments. The surgeon has the dilemma as to whether the patient should be deepened and returned to theatre, or fully awakened in the hope that the event is transient.
Presented with this dilemma, I consider the likelihood I have caused an unrecognised arterial injury, and I determine how closely related to extubation and awakening the bleeding occurred.
“Most epistaxes occurring in recovery are related to extubation and the awakening of the patient. These events are messy and cause significant concern, especially in inexperienced environments.”
If the patient is turning purple, pulling at the tube and I think my surgery was without significant error then I reassure everyone to press on with extubation and full awakening of the patient.
When this occurs, it does present a risk to the airway. I take a large bore Zoellner suction to carefully clear the nose and nasopharynx if I am able (I very rarely use packs; just nasal bolsters, but the patient may not be still enough to safely do this). An experienced anaesthetist and recovery team will make this a non-event.
Orbital breaches
Most minor orbital breaches (through periorbita) do not result in serious complications. Any patient with an orbital fat exposure should be advised to avoid nose blowing for two weeks following surgery, during which time gentle douching can help to maintain comfort. In more extensive exposures, any tethered edges should be decompressed / released as possible, and an orbital surgeon (sub-specialist ophthalmologist) consulted at the earliest opportunity.
Intraorbital bleeding
Cases of orbital or anterior ethmoid artery injury may be complicated by intraorbital (retrobulbar) haemorrhage causing ‘orbital compartment syndrome’. This is an emergency that may progress to optic nerve and retinal ischaemia with visual loss.
Clinically this initially presents with proptosis, ecchymosis, chemosis and - in awake patients - pain. Constant vigilance is required (and accessible eyes) to monitor for this complication during surgery. Where this occurs, any nasal packs should be removed, and a medial orbital decompression extended as required to allow any haematoma or ongoing bleeding to discharge into the nasal cavity.
Where the same occurs in recovery (which is less common), nasal packs should be removed, and the patient returned to theatre for exploration, decompression and / or control of bleeding. Where signs progress to ophthalmoplegia, visual / pupillary changes, or intraocular pressures >40mmHg, an emergency orbital decompression by lateral canthotomy and cantholysis is indicated.
Skullbase injury
These injuries carry a risk of meningitis, but antibiotics are only advocated if symptoms present. The risk is ongoing in persistent cases. If recognised intraoperatively, immediate repair is recommended - most are small, and easily closed with a fat plug and / or fascia graft secured by a mucosal graft or flap, fibrin glue, and nasopore pack. Larger defects are best supported by inclusion of a bone (e.g. turbinate) or cartilage graft. Conservative management includes bed rest, 15-30˚ head elevation, and avoidance of straining, lifting, or nose blowing but, in contrast to traumatic cases, this has little role, except perhaps while awaiting an experienced rhinologist to perform a repair.
Carotid injury [3]
It is beyond the remit of this manuscript to offer much advice on this type of injury with which we ourselves have thankfully little experience.
Immediate management
- Communication of the complication with the theatre team and additional assistance as required.
- Anaesthetic management of haemodynamics and fluid / blood resuscitation.
- Maintaining a field with twin large bore suctions, an endoscrub device, and a two man approach.
- Any procedure required for access (e.g. wide sphenoidotomy, turbinate lateralisation).
- Carotid occlusion by pressure in the neck may help to improve the surgical field.
Surgical control of bleeding
- Tamponade may be achieved through sphenoid packing (usually ribbon gauze) - this cannot be used if the dura has been opened. The technique may be easiest for those without extensive experience in endonasal surgery. Haemostatic measures, such as direct application of a muscle patch, can be used to achieve control, as described in the referenced article.
- Direct suture or u-clip closure may also be possible, but are always challenging.
- Cervical carotid ligation is not recommended - it carries a significant risk of cerebrovascular accident, may not control bleeding, and will prevent later endovascular access.
Neuroradiologic intervention (or imaging)
Immediate endovascular intervention is a final option (if available) in uncontrolled bleeding.
Even where this is not immediately required, it has a role in definitive management - stenting the injury or occluding the vessel to achieve long term control. It is no panacea; stenting the tortuous cavernous carotid may be impossible or cause further complications and, even in patients with good collateral circulation, occlusion carries a 5% risk of cerebrovascular accident.
Angiography is also required to exclude longer term complications such as false aneurism.
References
1. Barnes M, Surda P, Douglas R, Shao A. Functional Endoscopic Sinus Surgery (FESS) - Part 1. ENT and Audiology News 2016;25(3):104-7.
2. Hopkins C, Andrews P, Holy CE. Does Time to Endoscopic Sinus Surgery Impact Outcomes in Chronic Rhinosinusitis? Retrospective Analysis Using the UK Clinical Practice Research Data. Rhinology 53 2015;1:18-24.
3. Padhye V, Rowan V, Wormald PJ. Management of Carotid Artery Injury in Endonasal Sur-gery. International archives of otorhinolaryngology 18 2014;S02:173-8.
Declaration of Competing Interests: This article has been contributed to by SurgTech – a non-profit, open membership organisation established to promote open access surgical education. Our members are in no way financially reimbursed for their contributions.