Obstructive sleep apnoea (OSA) is one of the most common diseases in industrialised countries and is characterised by an intermittent obstruction of the upper airway during sleep. The standard treatment for OSA is continuous positive airway pressure (CPAP) therapy, which is limited by acceptance issues of 50%-70% [1]. Therefore an increasing number of patients remain vulnerable to the devastating consequences of untreated OSA.
A novel and minimally invasive treatment, selective hypoglossal nerve stimulation (Inspire® Upper Airway Stimulation, Maple Grove, USA), has emerged over recent years to overcome the obstacles associated with conventional anatomy altering surgeries. This treatment uses nightly stimulation at each inspiration to prevent upper airway collapse, by moving the tongue base and velum tissue anteriorly. Unlike the different variations of anatomy altering surgeries, implantation of the three components (IPG, stimulation lead, sensor) can be conducted in a standardised approach, which leads to a high success rate in properly selected patients.
Clinical evidence
Since an early report by Schwartz et al. in 2001, the method has been tested in multiple clinical trials [2, 3]. Most recently, the STAR trial was conducted as a pivotal study for examining safety and efficacy of the Inspire® Upper Airway Stimulation system.
The study was initiated in 2012, with the results published in the New England Journal of Medicine in January 2014 by Strollo et al. [3]. Median AHI in this cohort of 126 patients decreased from 29.3/h to 9.0/h at 12 months’ follow-up, which was associated with significant gains in health-related quality of life. Serious adverse events in only 2% of patients were rare. Unlike CPAP therapy, therapy adherence remained high with an every night usage in 86% of patients at 12 months.
Long-term outcomes of the STAR trial were published in November 2015 [4]. The results show a durable therapy effect with a mean AHI of 6.2/h, sustained improvements in health-related quality of life and a therapy adherence of 81% after 36 months’ follow-up.
Preliminary data from a post-market registry, which was initiated in Germany after CE mark approval, confirms the results of the STAR trial in a real-world setting.
Treatment path and clinical setting
Patient selection and screening is crucial for successful treatment with upper airway stimulation. The therapy is indicated for adult patients with an AHI from 15/h – 65/h.
An important contra-indication is complete concentric collapse at the level of the soft palate during drug-induced sleep endoscopy (DISE). In addition, patients should not have significant central or mixed sleep apnoeas (usually < 25% of total events). Overall efficacy in these patients can be limited, although the obstructive part of the disease is sufficiently treated.
Detailed preoperative planning is important to achieve optimal outcome for each patient. For planning of the neck incision, it is helpful to do a ultrasound of the submandibular gland upfront, to identify its anterior border. The incision is carried for 4cm anteriorly, about one finger below the mandible (Figure 1). To avoid bleeding during the tunnelling of the stimulation leading to the IPG pocket, it is recommended to mark the course of the external jugular vein. The incision for the IPG pocket is placed two fingers below the clavicle on the pectoral muscle. Sensor placement requires a third incision on the lateral chest wall, lateral to the inferolateral border of the pectoralis major muscle.
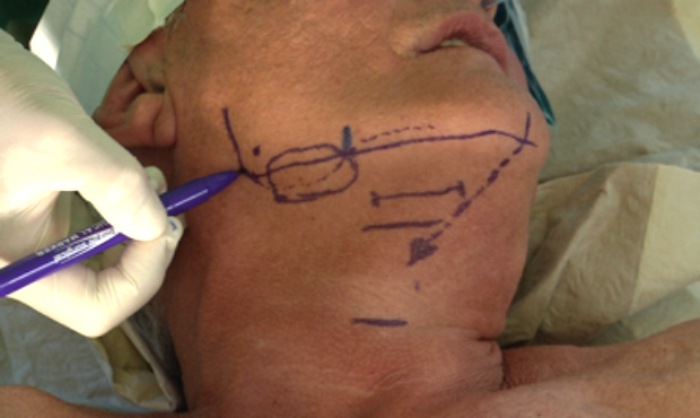
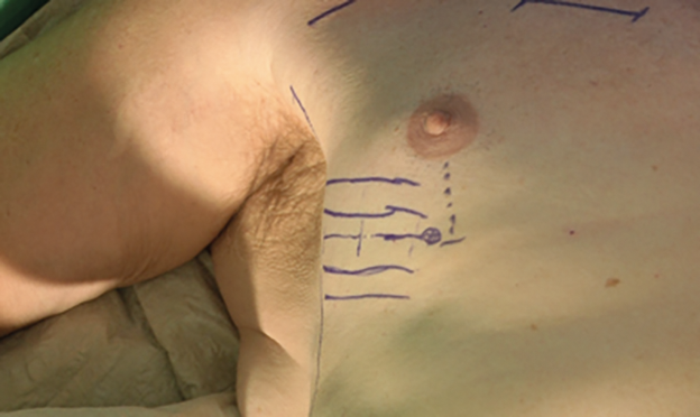
Figure 1. (Top) Preoperative planning of the neck incision, and
(Bottom) preoperative planning of the sensor incision.
Unhindered protrusion of the tongue base is crucial for achieving optimal clinical outcomes. This requires that only hypoglossal nerve fibres are recruited, that supply protrusor muscles (Figure 2).
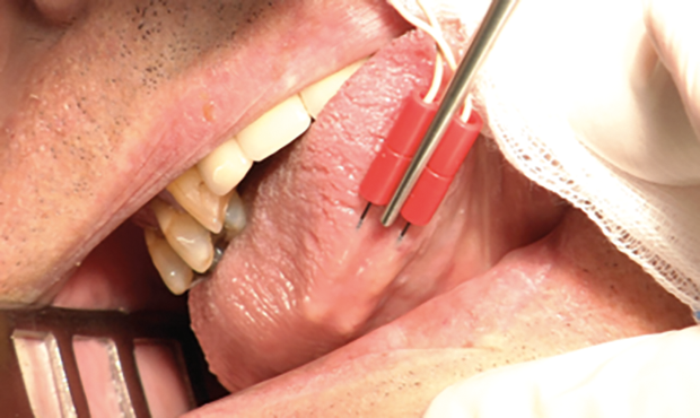
Figure 2a.
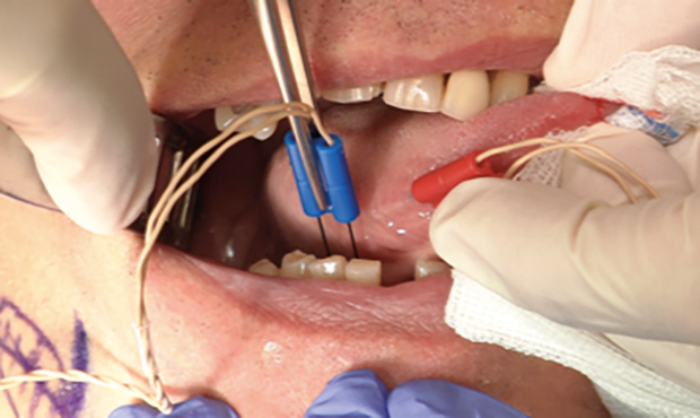
Figure 2b.
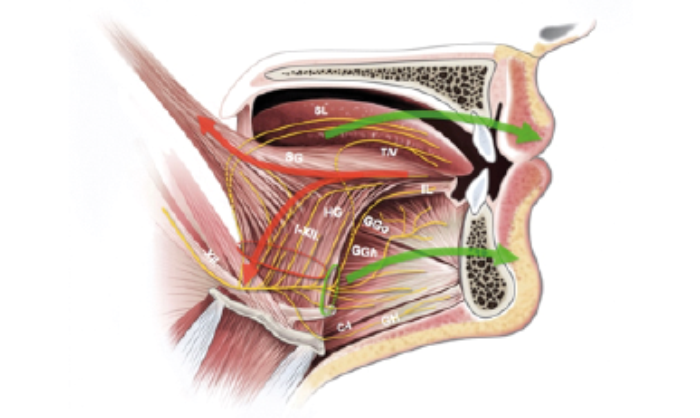
Figure 2c.
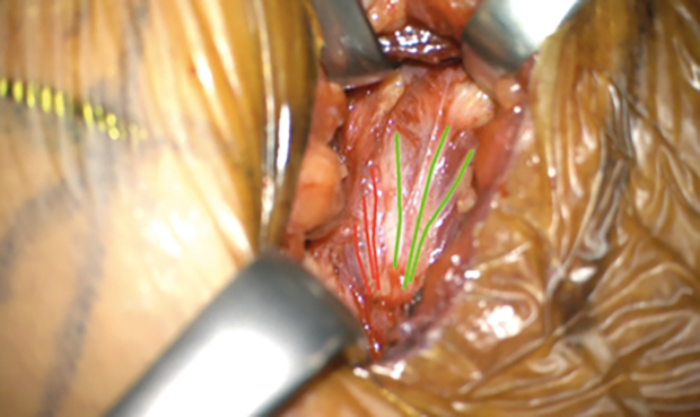
Figure 2d.
Figure 2. Placing of the NIM electrodes. (A) Red electrode shows the stimulus potentials of styloglossus and hypoglossus muscles, which need to be excluded as retractors; B) blue electrode shows the stimulus potentials of genioglossus muscle, which need to be included as protrusors. C) Schematic drawing of the hypoglossus nerve fibres and its supplying muscles. D) Intraoperative view of the nerve and its nerve branches (red are exclusion nerve fibres and green are inclusion nerve fibres).
The function of the branches can be tested with intraoperative neuromonitoring (NIM). Therefore two needle electrodes are placed in the oral cavity: one on the ventrolateral surface of the tongue to obtain signals from retractor muscles, and one in the genioglossal muscle for signals from the protrusor muscles. The hypoglossal nerve is developed in the lower border of the Lesser triangle. The accompanying ranine vein is ligated to avoid bleeding during dissection of the different branches. In a next step, the anterior border of the hyoglossal muscle is identified as a landmark for further preparation. Here the hypoglossal nerve main stem ramifies into the different branches that supply the intrinsic and extrinsic tongue muscles. To differentiate protrusor from retractor branches, the nerve response is evaluated with neuromonitoring (Figure 3).
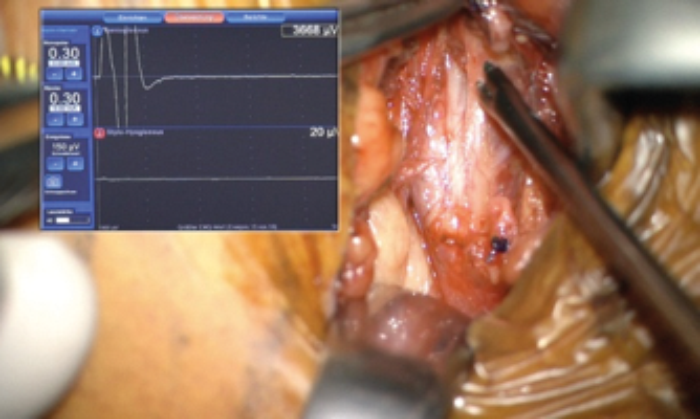
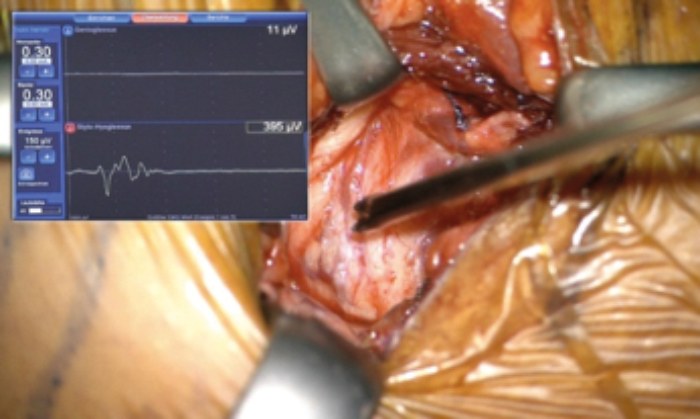
Figure 3 (Top and Bottom). Intraoperative NIM signal for the different stimulation of the nerve fibres.
The main branches that need to be recruited are genioglossus horizontal, genioglossus verticalis, transversalis and, if technically possible, the nerve which supplies geniohyoid muscle. This nerve runs along the hypoglossal nerve, though it originates from the ramus cervicales C1. Once all protrusor branches are identified, a window of 1cm width is created to place the cuff electrode. With the help of a right-angled dissector, the cuff is then wrapped around the hypoglossal branches. The electrode is then secured on the digastric tendon to avoid dislodgement during neck movements (Figure 4).
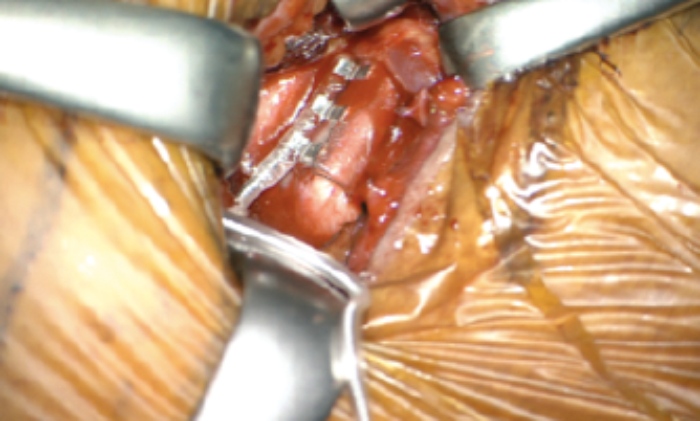
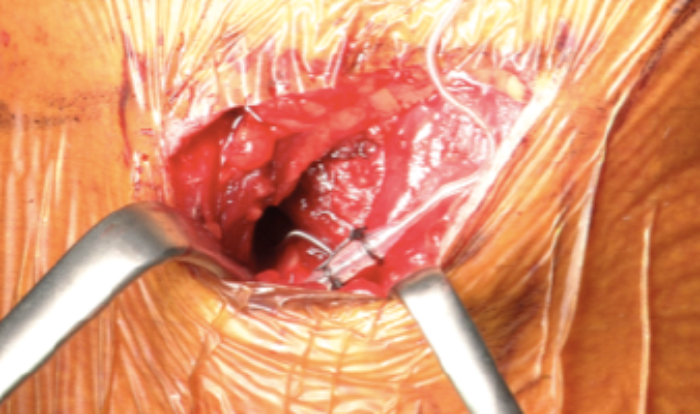
Figure 4 (Top and Bottom). Fixed cuff at the end of cuff placement.
As a next step, a 5.5 x 6cm subcutaneous pocket to host the IPG is created two fingers below the clavicle on the pectoral muscle (Figure 5).
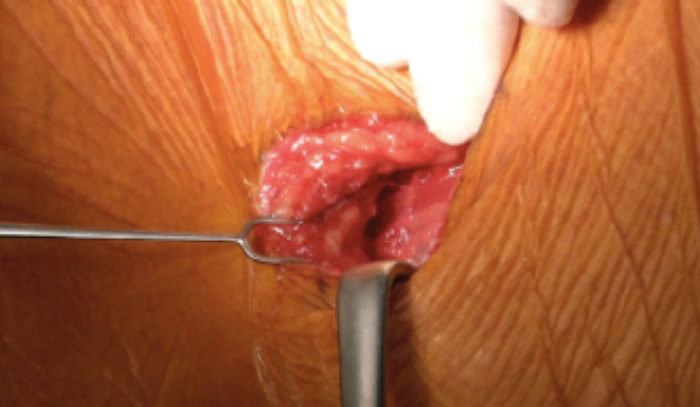
Figure 5. Incision of the IPG pocket.
Implantation of the sensor requires a 4cm incision on the lateral chest wall. After dissecting the subcutaneous tissue, the serratus muscle is split (divided) longitudinally to expose the intercostal space. A tunnel of 5cm length is then created to insert the sensor between the internal and external intercostal muscles. The sensor is secured at the fascia of the muscles after testing, to avoid dislodgement (Figure 6).
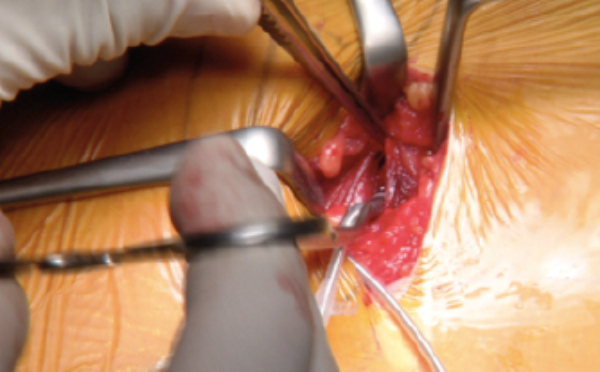
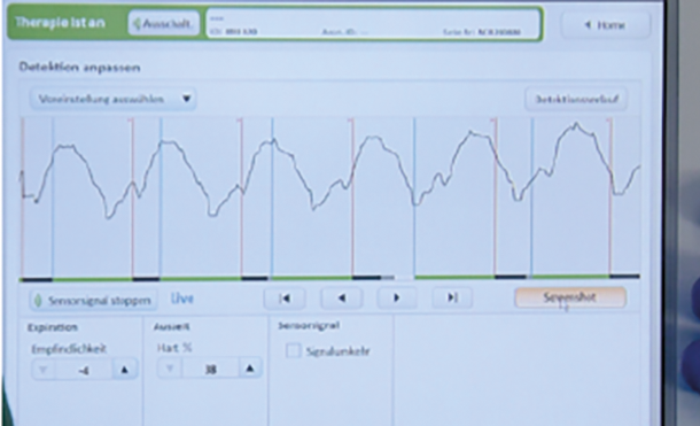
Figure 6: Sensor placement (Top) and its breathing signal (Bottom).
After subcutaneous tunneling of sensor and stimulation leading to the IPG pocket, both are connected to the IPG. Testing the system with regards to tongue motion and sensor signal quality closes the procedure (Figure 7).
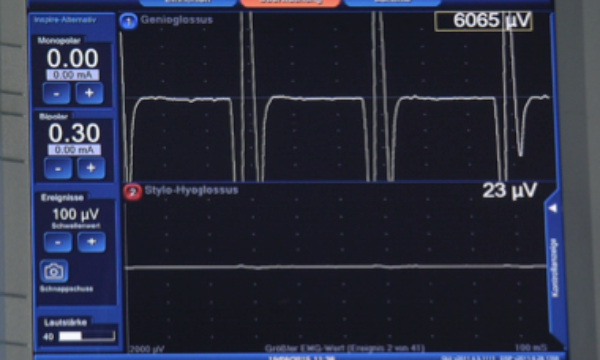
Figure 7. NIM signal during verification of the system.
All incisions are sutured in standard technique, without use of drains to reduce the risk of postoperative infections. Typically patients can be discharged on the second or third postoperative day, with only minimal need for oral pain medications. The system is activated with standard settings four weeks after implantation. After another four weeks of training with the system, titration and individualisation of stimulation is done within an overnight stay in the sleep lab. Once set-up, patients are checked annually for therapy efficacy, adherence and battery status.
References
1. Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. The European Respiratory Journal 2011;37:1000-28.
2. Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnoea. Arch Otolaryngol Head Neck Surg 2001;127:1216-23.
3. Strollo PJ, Jr., Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnoea. N Engl J Med 2014;370:139-49.
4. Woodson BT, Soose RJ, Gillespie MB, et al. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnoea: The STAR Trial. Otolaryngol Head Neck Surg 2016;154:181-8.
Declaration of Competing Interests: Dr Clemens Heiser is the study investigator and consultant of Inspire Medical Systems and received personal fees, travel expenses and research grants (third-party funds).





