Do the potential side-effects on hearing and tinnitus need to be a ‘necessary evil’ of platinum-based chemotherapy? How strong is our evidence base when offering advice to patients and fellow clinicians? David Baguley and his team from the University of Nottingham explore the subject.
Platinum-based chemotherapy, most commonly involving cisplatin or carboplatin, is used to treat common cancers in adults - such as breast, ovarian, and testicular tumours - and brain tumours in children. Whilst the efficacy of such treatments has made a substantial contribution to improved outcomes and life expectancy [1], such drugs are associated with significant ototoxicity, resulting in iatrogenic hearing loss and tinnitus.
These adverse effects have previously been viewed as a ‘necessary evil’ in attempts towards curative treatment, but with increased survival rates has come a new concern about the impact of such symptoms upon quality of life in survivorhood. Figure 1 shows a drawing by a patient of their tinnitus during cisplatin chemotherapy, indicating the coruscating intensity of their tinnitus.

Figure 1. A drawing by a patient of their tinnitus during cisplatin chemotherapy,
indicating the coruscating intensity of their tinnitus.
The following scenario may help to frame the clinical questions:
A busy morning clinic, and your next patient raises some challenging issues. She is a 24-year-old woman undergoing platinum-based chemotherapy for advanced ovarian cancer. After cycle three of a planned six, she has developed a mild bilateral high frequency hearing loss and severe bilateral high frequency ‘hissing’ tinnitus: she is acutely distressed, is not sleeping, and feels that all is lost. Her relationship with her fiancé is deteriorating due to her irritability and distress, and a planned house move has been indefinitely postponed. Her question to you is specific: will further chemotherapy worsen her already intolerable tinnitus? Oncology colleagues have indicated that you may be able to supply an answer, but are concerned about possible treatment non-compliance, which would substantially reduce their aim of a curative outcome. Do you have data to provide answer based on evidence?
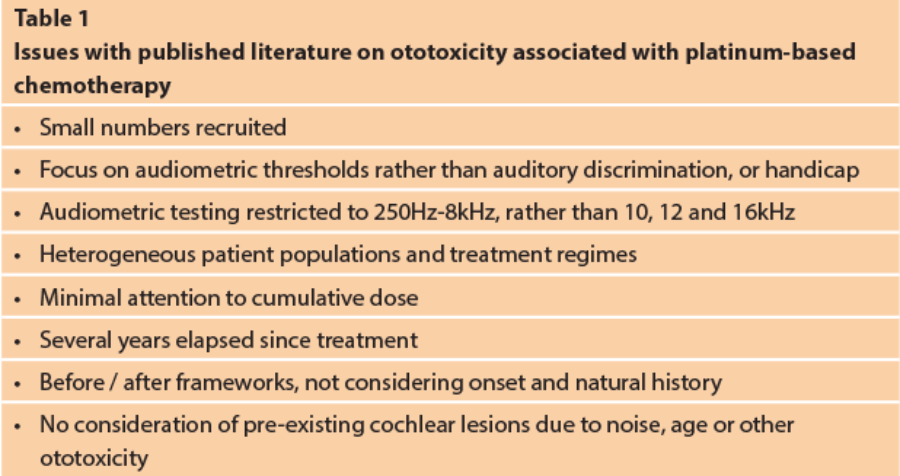
Whilst there are many scientific papers indicating that cisplatin in particular can be associated with a progressive, high frequency cochlear hearing loss, and an incidence of troublesome tinnitus, the quality of the literature is not high. Many of the studies are observational and retrospective, and Table 1 indicates various problems that can been identified.
The answer to the question posed thus has to be provisional, potentially along the lines of:
‘It is hard to predict whether further chemotherapy will worsen your tinnitus, though anecdotally, patients who do acquire tinnitus in this way don’t commonly speak about it worsening and worsening. Stopping the chemotherapy is also likely to lessen the effectiveness of the treatment by a substantial amount, but your oncologist will be able to better quantify this. I can certainly try to help you in lessening the effect of the tinnitus with support and devices, if needed.’ There are undoubted and substantial challenges in undertaking robust research in this area. Patients undergoing chemotherapy are often anxious and vigilant to new symptoms or side-effects.
Many may already have been experiencing trouble sleeping before the onset of any tinnitus, so if / when tinnitus begins, they may have very limited resilience with which to deal with it. As such, the chemotherapy patient group is almost primed for tinnitus to develop into a significant issue, though the countervalent factor may be that tinnitus would be only one of several, perhaps many, physical and emotional challenges. All of these, along with other issues, may influence reaction to tinnitus and hearing loss. Another challenge in research is that, whilst much chemotherapy practice is protocol-based, divergence from protocol is common as treatments may be delayed, modified, or added to in the case of particular circumstances. Thus, the identification of a truly homogeneous treatment group may be difficult.
There are indications, however, that a more systematic approach is being taken with this topic by several groups worldwide. The Platin Study Group is an international, multicentre and multidisciplinary collaboration, with a specific focus upon adult survivors of testicular cancer treated with cisplatin, and is taking a comprehensive approach to their large-scale retrospective dataset regarding the incidence of hearing loss and tinnitus. Recent work has indicated that in a retrospective study of 488 adult survivors of testicular cancer, 18% of the patients tested had a severe / profound hearing loss, and a further 37% a moderate loss, though only 1.4% were hearing aid users [2]. Furthermore, 15.8% experienced intrusive tinnitus. The unmet demand for audiology / otology care in this patient population is substantial.
International guidelines indicate that regular audiometric testing should be undertaken to monitor hearing in such at-risk populations, with the aim of the early detection of iatrogenic hearing loss. Whilst empirical evidence of compliance with such guidelines has not been identified, indications are that the implementation of audiometric monitoring is patchy [3].
Some other evidence is also emerging. Some clinical research groups are starting to consider long-term impacts and outcomes of hearing loss and tinnitus in cancer survivorhood [4]. Factors influencing susceptibility to ototoxicity associated with platinum-based chemotherapy are being investigated, including cumulative cisplatin dose, and patient factors such as genetics, age, gender, body mass index, and nutritional status [3]. Cisplatin has been detected in plasma 20 years post-treatment [5]; this may lead to an ongoing cochlear vulnerability to age-related cochlear degeneration, and to other cochlear insults associated with exposure to noise, or other ototoxic agents or drugs. Considerable effort is being undertaken in the development of otoprotective agents, and there is some early evidence of benefit from clinical trials [6].
The Clinical Hearing Sciences group of the NIHR Nottingham Biomedical Research Centre is undertaking research with the aim of investigating methods of early detection of ototoxicity; detailing the long-term impacts of ototoxicity in adult survivors of cancer; and performing clinical trials of interventions. The team lead is Professor David Baguley, appointed in October 2016 after 30 years of clinical leadership in audiology and tinnitus research at Cambridge University Hospitals, and the wider team includes oncologists (clinical and academic), otologists, audiologists, cancer and research nurses, pharmacists, and research scientists.
Our aim in Nottingham is to use our multidisciplinary community to bring insights and perspectives from clinical audiology / otology, auditory neuroscience and oncology to bear upon ototoxicity associated with platinum-based chemotherapy in adult survivors of cancer. Public and patient involvement in the design and instigation of the research programme is of fundamental importance.
The initial scoping reviews have been funded by the British Tinnitus Association, and the findings are being used to shape the research theme as grant applications are underway. National and international collaborations are being developed, with open discussions with colleagues in the Platin Study Group and at University College Hospital, London.
References
1. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7(8):573-84.
2. Frisina RD, Wheeler HE, Fossa SD, et al. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol 2016;34(23):2712-20. 3. Paken J, Govender CD, Pillay M, Sewram V. Cisplatin-Associated Ototoxicity: A Review for the Health Professional. J Toxicol 2016;2016:1809394.
4. Skalleberg J, Solheim O, Fosså SD, et al. Long-term ototoxicity in women after cisplatin treatment for ovarian germ cell cancer. Gynecol Oncol 2017;145(1):148-53.
5. Oldenburg J, Gietema JA. The sound of silence: A proxy for platinum toxicity. J Clin Oncol 2016;34(23):2687-9.
6. Freyer DR, Chen L, Krailo MD, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18(1):63-74.








