Global awareness of cochlear implants as a solution for hearing loss is slowly increasing and gaining acceptance. The potential for combining cochlear implants with inner ear therapeutics is immense, with promise in several areas. This article takes us on a whistlestop tour through this theme, planting seeds for the future of cochlear implants and inner ear therapeutics, for hearing protection and restoration.
The cochlear implant (CI) has been transformative in the treatment of hearing loss and is widely touted as the most successful sensory or neural prosthesis. For over 30 years, CIs have been implanted in both children and adults with significant hearing loss to restore the sensation of hearing via direct electrical stimulation of the auditory nerve (see Figure 1).
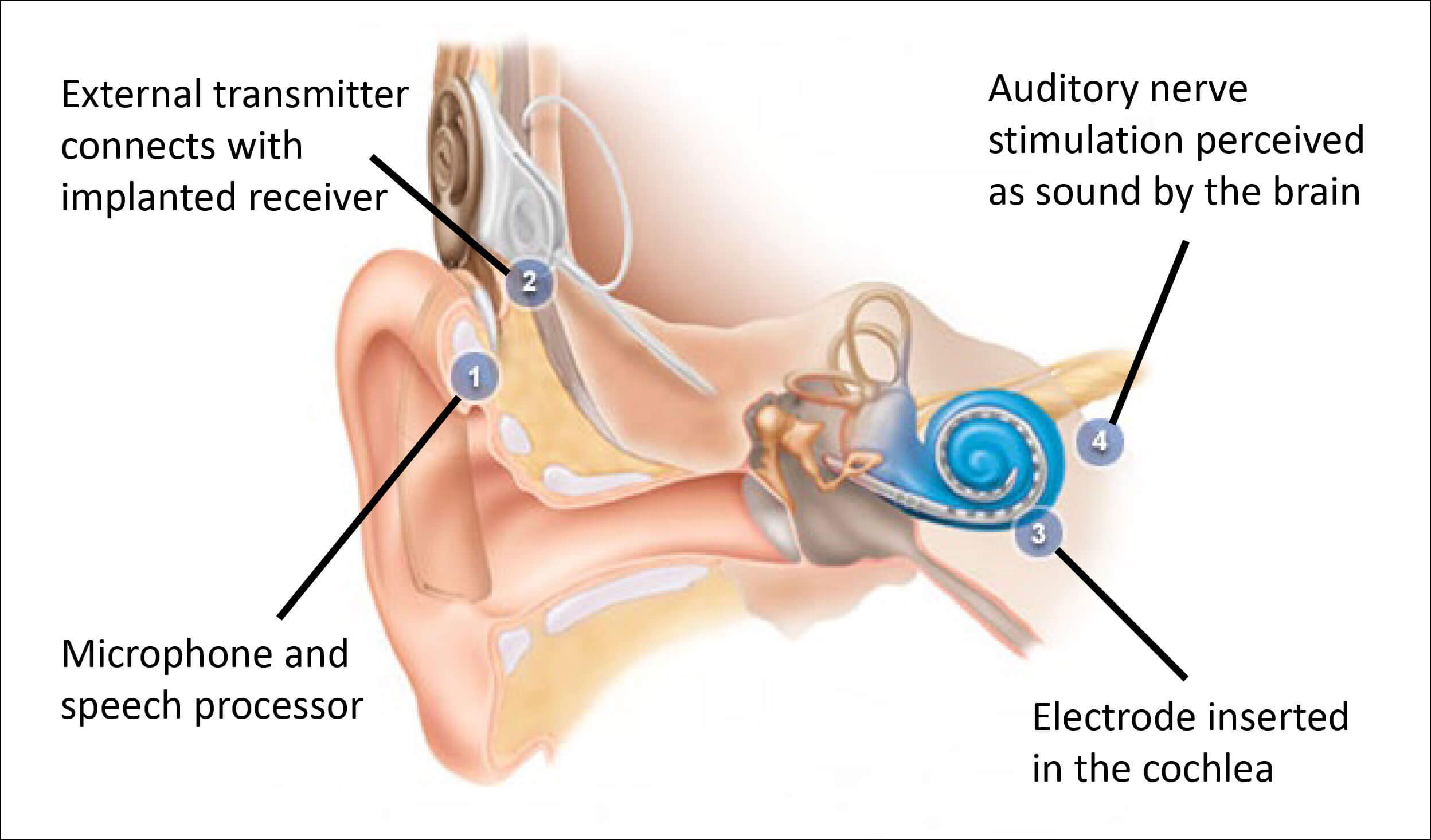
Figure 1. How cochlear implants work.
Despite advances in both electrode design and surgical techniques, barriers remain in the uptake of CIs globally [1]. Hearing aids, typically indicated for more moderate forms of hearing loss, provide amplification of sound but do not address the underlying and evolving biological circumstances of hearing loss.
Given the staggering numbers of individuals affected and impacted by hearing loss [2], it is unsurprising that the pharmaceutical and investment sectors have recently turned their gaze towards this field, stimulating the development of therapeutics beyond academia. But the road to developing a therapeutic for hearing loss has demonstrably proven a bumpy one and, despite the continued hope abounding, many questions remain around how and when these therapeutics will be in use.
Therapeutics for hearing loss
As has been covered extensively elsewhere [3], specific approaches to developing a therapeutic for hearing loss are varied across mechanism of action (MOA), delivery method and target patient population. Shown in Figure 2, many therapeutics remain in preclinical development, whilst a select few have progressed to clinical studies. Broadly, therapeutics for hearing loss fall into two main categories, (which are further subdivided): 1. Protective - to guard the delicate sensory cells of the inner ear against damaging agents such as noise or drugs; and 2. Restorative - harnessing gene therapy, cell replacement, or regenerative compounds to restore the function of the cochlea, auditory nerve, or central processing capability.
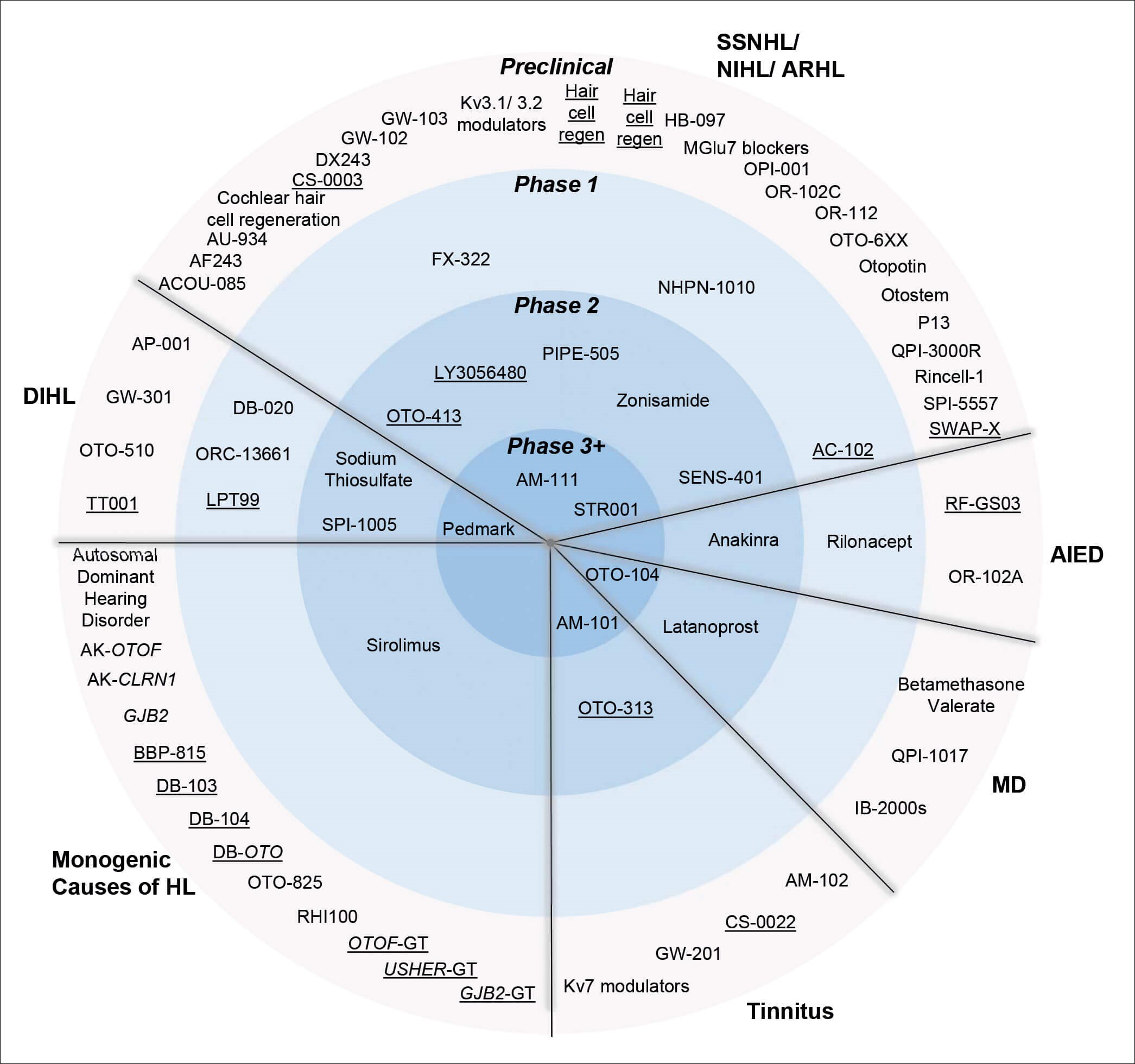
KEY
HL Hearing Loss
DI Drug Induced
NI Noise Induced
AR Age Related
SSN Sudden Sensorineural
AIED Autoimmune Inner Ear Disease
MD Méniѐre’s Disease
Figure 2. The global therapeutic pipeline for inner ear disorders taken from [3] with author permission.
Although promising preclinical data has provided a much-needed stimulus to the field, leading to investments and start-ups, a steep decline in therapeutics moving through the clinical pipeline is apparent. Whilst this is not unusual in drug development in general, here we call out some of the particular challenges to the progress of hearing loss therapeutics and how they may be ameliorated by combination with CIs.
Key barriers to developing inner ear therapeutics – can CIs help?
The first significant barrier faced by the field is translation. Hearing loss, particularly when acquired in adulthood, is a complex, and often multifactorial condition. As such, it is a major challenge to replicate the human condition in preclinical animal models [3]. Without predictive preclinical models the risks associated with moving into clinical trials are high. Beyond the difficulties in producing preclinical models, the lack of precision in defining the pathophysiological basis of the different forms of hearing loss in patients results in an ambitious task to diagnose and recruit the target patient population to a clinical study. Creating a largely homogenous group of study participants is a key factor in being able to interpret results. CIs offer attractive solutions – preclinical models of insertion trauma are predictive of the human response; the CI can be utilised for objective measures or diagnostics from preclinical models through to the clinical setting; and the target patient population for CI is both better characterised and easier to recruit to than some forms of hearing loss currently being explored in a clinical setting e.g. sudden sensorineural hearing loss.
The next major barrier to the development of therapeutics for hearing loss is the lack of objective measures [3]. A number of recent clinical studies for hearing loss therapeutics employed subjective speech (or word) in quiet or noise tests as a primary measure, and failed to see successful, efficacious outcomes. Whilst there may be many other factors at play, the reliability of subjective measures, where the patients themselves report the results, certainly in the absence of corroborating objective measures, is a major hurdle to the field. Here again, CIs could be harnessed to provide these much-needed objective measures as these devices have incorporated an e-physiology suite which can measure aspects such as: the status of the electro neural interface, the approximate number of surviving cells in the spiral ganglion, peripheral neural function, and monitoring of cochlear and auditory nerve function.
“By combining appropriate therapeutics with CIs, there is the potential to augment patient outcomes and deliver a more powerful intervention”
A third key hurdle is the effective delivery of a therapeutic to its target tissue or cell. In the early days of pharma interest in the hearing loss space, the preferred delivery approach was systemic or oral dosing. Whilst a simple and accessible form of administration, systemic delivery is not ideal for hearing loss due to the challenges of a molecule reaching the cochlea and doing so in efficacious concentrations, all without causing off-target effects [4]. Recently, companies in this space have pivoted to a more local delivery approach, for example intratympanic injection of a therapeutic through the ear drum in an excipient gel. This method of administration may also be suboptimal as it relies on the properties of the therapeutic to enter and diffuse (passively) along the cochlea, providing efficacious distribution and exposure. Whilst the least invasive means for delivering an intervention should always be considered first, the cochlea represents a unique target due to its tiny size, location within the strongest bone of the skull, and delicate and complex structure.
The burgeoning therapeutics field should look to the CI space and harness the decades of expertise gained in accessing the cochlea as atraumatically as possible. Therapeutics may be delivered in conjunction with a CI at time of surgery, ensuring they will reach the intended target, or may be delivered ahead of CI surgery with the opportunity to sample the cochlear fluid (perilymph) during the surgery. Analysis of these samples can provide much needed evidence for the therapeutic reaching its target if delivered systemically or intratympanically.
In addition to the above, the CI indication should provide an enticing space in and of itself for pharmaceutical development. By combining appropriate therapeutics with CIs, there is the potential to augment patient outcomes and deliver a more powerful intervention. Although the size of the total addressable CI market, if limiting to severe-to-profound hearing loss, is far smaller than mild to moderate, it still reaches above 60 million people worldwide [2]. This number may increase year on year with ageing populations. Indeed, the main CI companies have, in recent years, partnered with and/or invested in biotech companies and academic research groups to identify therapeutics to augment CI outcomes.
Treatments for hearing loss, today and tomorrow
The burden of hearing loss is vast and is felt at the individual and societal level [2]. Those affected are looking to the ENT, audiology, device, and pharmaceutical spheres for more, better, and different solutions. Recent studies linking hearing loss to dementia [5] further underpin the great need for better treatments whist also highlighting the potential dangers of waiting to adopt current interventions. Cochlear implants, and hearing aids, can markedly improve the quality of life of individuals with hearing loss and it is hoped the entrance of therapeutics will, in the future, build upon this legacy.
References
1. Bierbaum M, McMahon C, Hughes S, et al. Barriers and Facilitators to Cochlear Implant Uptake in Australia and the United Kingdom. Ear and Hearing 2020;41(2):374‑85.
2. World Health Organization. World Report on Hearing. Geneva, Switzerland. 2021.
www.who.int/publications/i/
item/world-report-on-hearing
Last accessed August 2022.
3. Isherwood B, Goncalves AC, Cousins R, Holme R. The global hearing therapeutic pipeline: 2021. Drug Discov Today 2021;27:912-22.
4. Cousins R. Hearing loss drug discovery and medicinal chemistry: challenges and opportunities. Progress in Medicinal Chemistry 2022;61:1-91.
5. Livingston G, Summerland A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390(10113):2673-734.