One of the headline speakers, Mark Zafereo, will be talking at IFOS about thyroid cancer. We hear from his team about some of the areas they will be discussing.
Locally advanced thyroid cancer generally refers to patients who have significant primary tumour extension beyond the margin of the thyroid gland to involve or threaten surrounding structures, or extracapsular extension of involved lymph nodes to invade nearby structures. Involvement of recurrent laryngeal nerves, trachea, laryngeal framework, and oesophagus can significantly affect the morbidity of surgery and a patient’s resultant quality of life. Additionally, these locally advanced thyroid cancers are associated with high risk for distant metastases, and often present with distant disease at diagnosis.
Until recently, nonsurgical therapeutic options for advanced thyroid cancer have been limited. Classical cytotoxic chemotherapy and external beam radiotherapy have limited efficacy for gross thyroid disease and are associated with significant toxicity. Over the last decade, there has been an exciting increase in understanding of the molecular mechanisms underlying thyroid carcinogenesis and in the development of therapeutics to target the tyrosine kinase pathway. Although these targeted therapies have side effects and toxicities which must be considered, they have demonstrated efficacy, opening up new therapeutic treatment options. Surgery remains an important therapeutic option for patients with locally advanced thyroid cancer, but the role of surgery is currently being reconsidered in light of these novel therapeutics.
Medullary thyroid cancer
All germline mutated medullary thyroid cancer (MTC), and approximately 50% of sporadic MTC is associated with a RET mutation. Selpercatinib and pralsetinib are RET-specific inhibitors which were FDA-approved in the United States for RET-altered thyroid cancer in 2020. In multicentre clinical trials, selpercatinib and pralsetinib demonstrated overall response rates of 73% and 71% for treatment-naïve patients, respectively [1,2]. However, long-term disease control on RET-specific inhibitors is uncertain, and there have been reports of resistance and progression in a minority of patients. Surgery has long been considered a mainstay of upfront treatment for patients with MTC. The advent of RET-specific drugs has renewed interest in the possibility of targeted therapy prior to surgery for patients who present with locally advanced MTC. ‘Selpercatinib Before Surgery for the Treatment of RET-Altered Thyroid Cancer’ (NCT04759911) is a phase II multicentre trial studying the impact of a RET-specific inhibitor given before surgery to measure, among other aims, the changes in expected and actual surgical morbidity/complexity associated with surgery for locally advanced MTC.
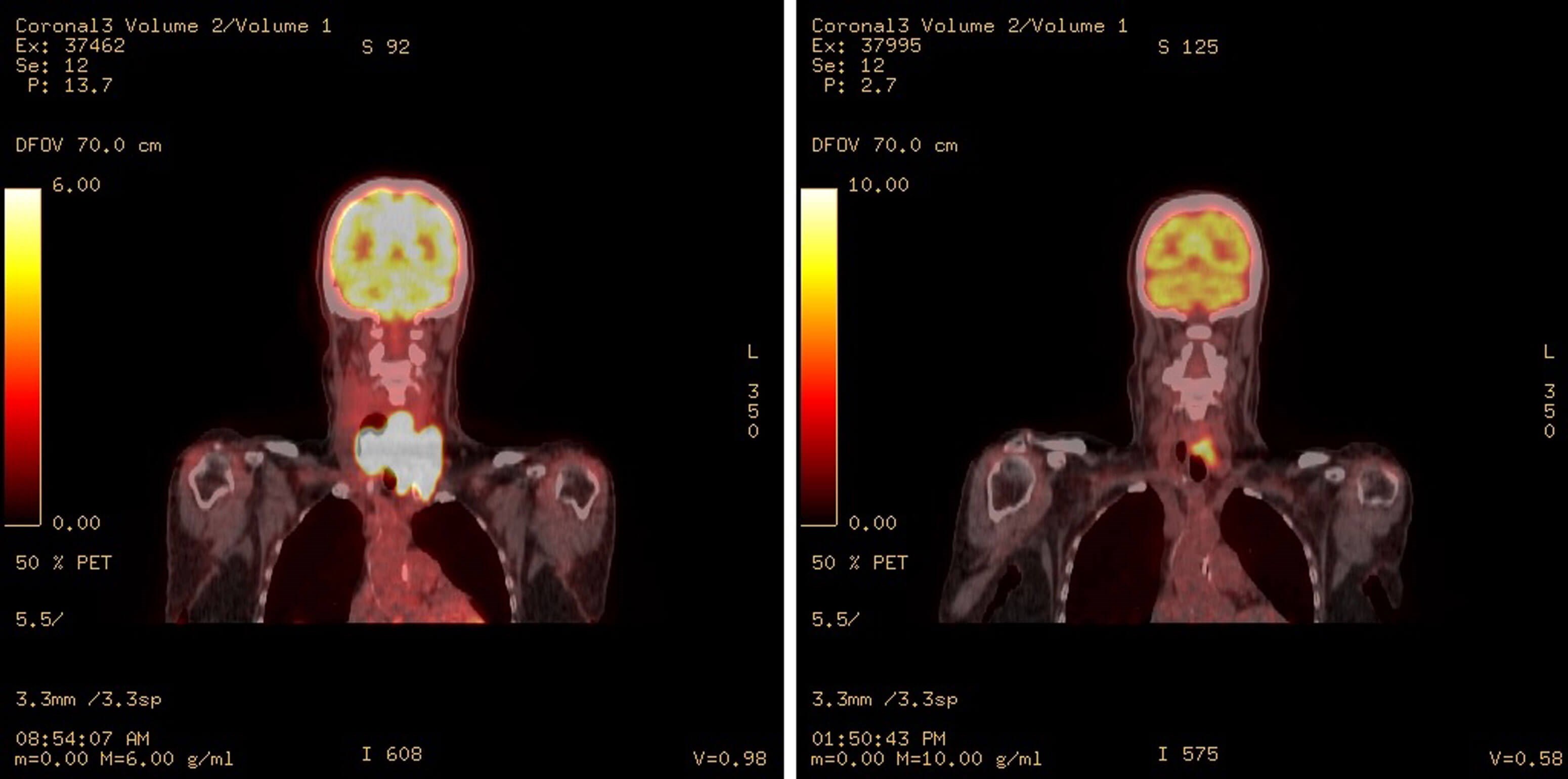
Figure 1. PET/CT coronal image of patient with BRAF V600E mutation treated with pembrolizumab (anti PD1), dabrafenib (BRAF inhibitor), and trametinib (MEK inhibitor). Left image shows intense hypermetabolism (SUV max 32) with a locally extensive and invasive anaplastic thyroid cancer before treatment. Right image shows same patient after two months systemic treatment and before surgery.
Anaplastic thyroid cancer
While only approximately 40% of anaplastic thyroid cancers have a BRAF V600E mutation, the dramatic and rapid effects of BRAF/MEK inhibition in this patient population have greatly changed the treatment landscape and prognosis for this subset of patients with ATC. A 69% overall response rate was achieved in a BRAF-V600E mutated population with locally advanced or metastatic ATC treated with dabrafenib and trametinib [3]. Immunotherapy added to BRAF/MEK inhibitor can be considered, especially for patients with PD-L1-positive tumours, with remarkable results in select cases (Figure 1). Furthermore, complete surgical resection following BRAF/MEK inhibitor and immunotherapy in BRAF(V600E)-mutated anaplastic thyroid carcinoma has been associated with favourable survival and functional outcomes [4,5]. ‘Pembrolizumab, Dabrafenib, and Trametinib Before Surgery for the Treatment of BRAF-Mutated Anaplastic Thyroid Cancer’ (NCT04675710) is a multicentre phase II trial investigating the effect of BRAF/MEK inhibition and immunotherapy before surgery for BRAF V600E-mutated anaplastic thyroid cancer. In addition to evaluating survival, a primary objective of this study is to demonstrate the extent to which this neoadjuvant triplet therapy changes surgical resectability and extent of surgery.
Differentiated thyroid cancer
While the vast majority of differentiated thyroid cancers (DTC) are curable with surgery alone, a small minority of DTCs represent biologically aggressive disease. These tumours may behave more like poorly differentiated disease, with faster growth, greater local invasion, and distant metastases; and are often associated with unfavourable mutational profiles (such as the combination of BRAF and TERT) and PET avidity. Neoadjuvant targeted therapy prior to surgery may be considered in select patients with locally advanced DTC in order to lessen the local disease burden, treat distant disease, buy time prior to surgery, or any combination of these reasons. For biologically aggressive DTCs with targetable mutations, such as those with BRAF V600E mutations, RET fusions, or NTRK fusions, respective drugs targeting these mutations may be considered; while for biologically aggressive DTCs without targetable mutations, nonspecific multikinase inhibitors such as lenvatinib may be considered. ‘Lenvatinib in Locally Advanced Invasive Thyroid Cancer’ (NCT04321954) is a phase II multicentre trial studying the impact of neoadjuvant lenvatinib for locally advanced DTC.
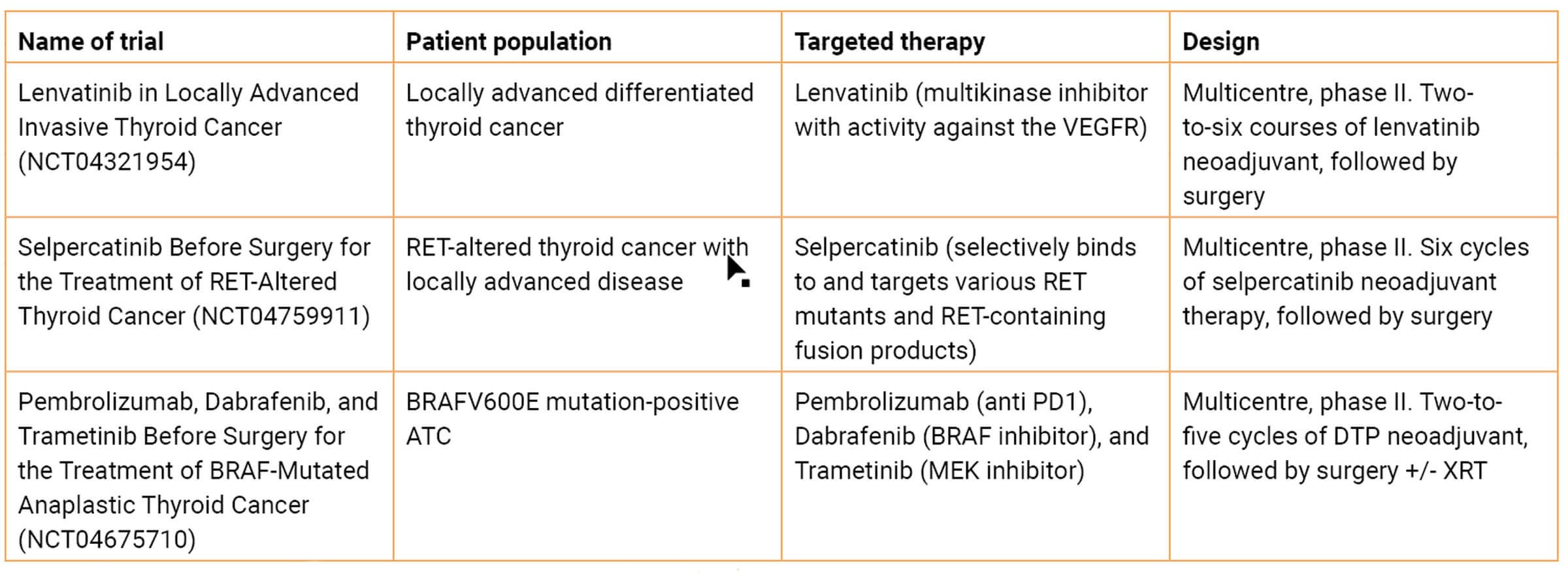 Table 1. Select advanced thyroid cancer neoadjuvant clinical trials.
Table 1. Select advanced thyroid cancer neoadjuvant clinical trials.
Locally advanced thyroid disease: current neoadjuvant targeted therapy clinical trials
Current clinical trials are integrating mutation-driven targeted systemic therapy and surgery to study impact on surgical resectability, quality of life, locoregional disease control, and survival in advanced thyroid cancer (Table 1). Targeted therapies have redefined the therapeutic approach to advanced thyroid malignancies. Many questions are yet to be answered, but the advent of molecular therapies has revolutionised the field. Future advances will not only determine optimal regimens for targeted therapy but will also delineate the role of such approaches across the continuum of multidisciplinary therapy for thyroid malignancies.
References
1. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020;383(9):825-35.
2. Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 2021;9(8):491-501.
3. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 2018;36(1):7-13.
4. Wang JR, Zafereo ME, Dadu R, et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAF(V600E)-mutated anaplastic thyroid carcinoma. Thyroid 2019;29(8):1036-43.
5. Maniakas A, Dadu R, Busaidy NL, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncology 2020;6(9):1397-404.