Matthew Smith discusses a project looking at outcomes of acute otitis externa interventions, and how, going forward, the INTEGRATE team are working with patients to develop outcome measures.
Acute otitis externa (AOE) is one of the most common conditions of the outer ear, requiring management in primary or secondary care. There has been little research into treatments for AOE, despite its high incidence and demands on healthcare resources.
Issues with designing studies relating to AOE have been a lack of widely accepted diagnostic criteria, and no standardisation of the all-important outcomes assessed in AOE interventional trials. In particular, there has been little or no consideration as to what patients feel is important when looking at the effects of new treatments.
For interventional studies to be relevant to clinical practice and policy makers, the reported outcomes must be important to all key stakeholders. Furthermore, these outcomes require widespread and consistent adoption to facilitate meta-analysis.
Core outcome sets (COS) are agreed standardised sets of outcomes that represent the minimum that should be measured and reported in all clinical studies of a specific condition [1]. The validity of a COS depends on its development, which must include working with key stakeholders to prioritise what may be a large number of candidate outcomes. The Core Outcome Measures in Effectiveness Trials (COMET) initiative publishes guidance on COS development [1], which is recognised internationally as best practice.
“There has been little or no consideration as to what patients feel is important when looking at the effects of new treatments”
To facilitate future research into AOE, INTEGRATE, the UK ENT Trainee Research Network set out to develop a COS to be used for adults with AOE, and to establish diagnostic criteria for AOE.
Study methodology
A three-stage methodology based on COMET recommendations was used. Further details of this and the study results can be found in our published manuscript [2].
Stage 1: Outcomes derived from patients
Former patients representing a broad demographic were surveyed using semi-structured interviews to understand their experiences regarding AOE and their opinion as to how treatment success should be measured. Candidate outcomes for the COS were then extracted from interview transcripts.
Stage 2: Comprehensive literature review
To capture all previously adopted diagnostic criteria and outcomes for AOE, an English-language literature search was performed for all studies reporting the effectiveness of any intervention for AOE in adults. Diagnostic criteria and outcomes were then extracted from relevant articles.
Stage 3: Stakeholder consensus process
Candidate outcomes from patients and the literature were grouped and, along with candidate diagnostic criteria, were included in a three-round online modified Delphi process, to establish consensus amongst key stakeholders [3]. Stakeholder groups were classified as either professionals or patients, with the professionals group comprising: consultant otologists and other ENT surgeons; ENT registrars; junior doctors and specialist nurses in ENT; general practitioners; microbiologists; and audiologists. Stakeholders were asked to anonymously rate their agreement with each item on an interval scale, and consensus to include an outcome or diagnostic item was assigned using pre-defined criteria. The Delphi process is outlined in Figure 1. A steering committee analysed the Delphi results and summarised these into a COS and clinically-relevant diagnostic criteria.
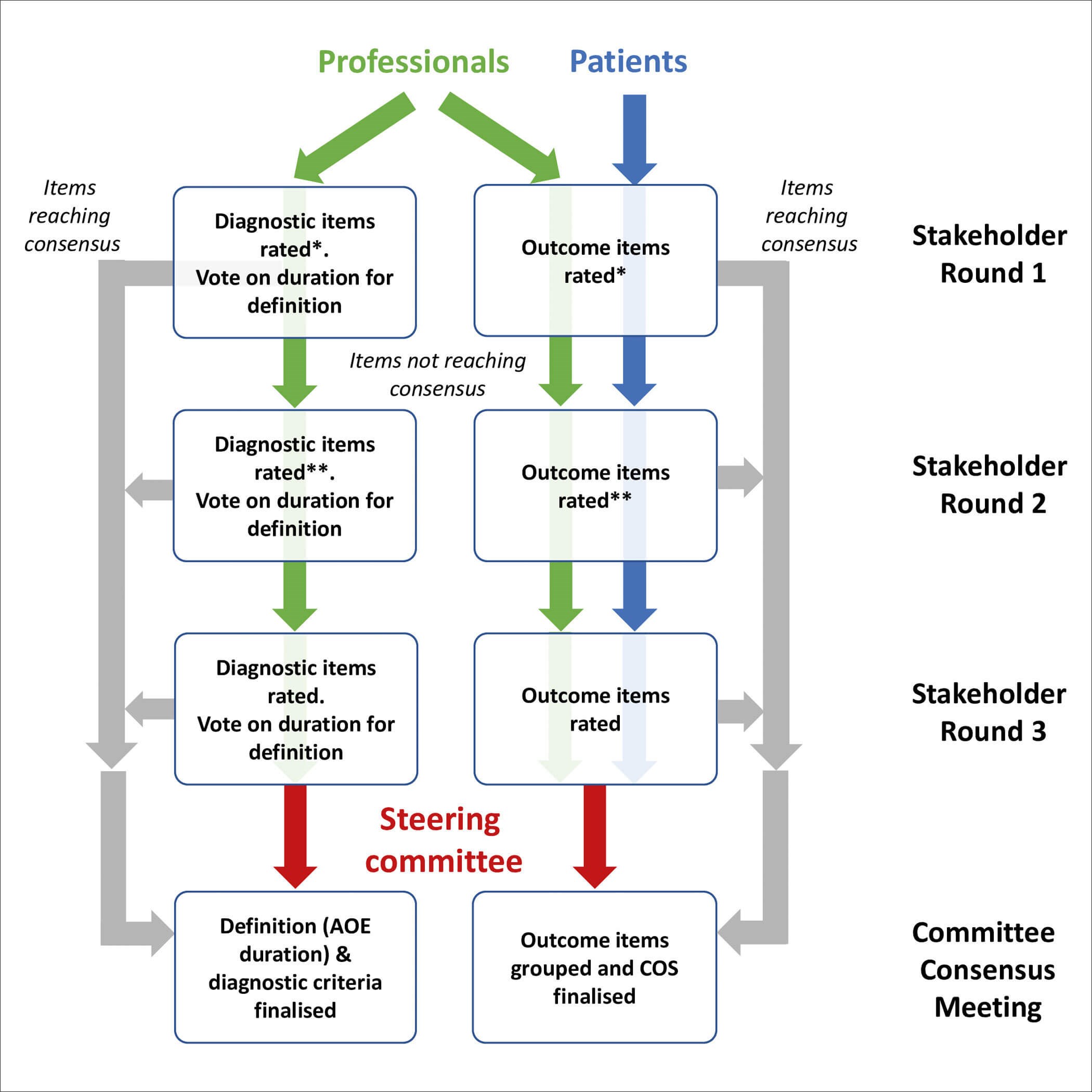
Figure 1. Flow diagram showing the three-stage Delphi consensus process [2].
Study results
Candidate COS items from patients (28 outcomes) and the literature (46 articles, 25 outcomes) were de-duplicated and amalgamated to a final candidate list (n=46). Patients emphasised the importance of quality-of-life and the impact of AOE on daily activities and work, for example stating, “I didn’t even leave the house for the first week because it smelt awful” and “I kept worrying I would be fired”. Via the Delphi process, stakeholders agreed on 31 candidate items, and the final COS covered six outcome domains, shown in Figure 2. Fourteen candidate diagnostic criteria were identified and eight reached inclusion consensus. The consensus diagnostic criteria can be found in our publication [2].
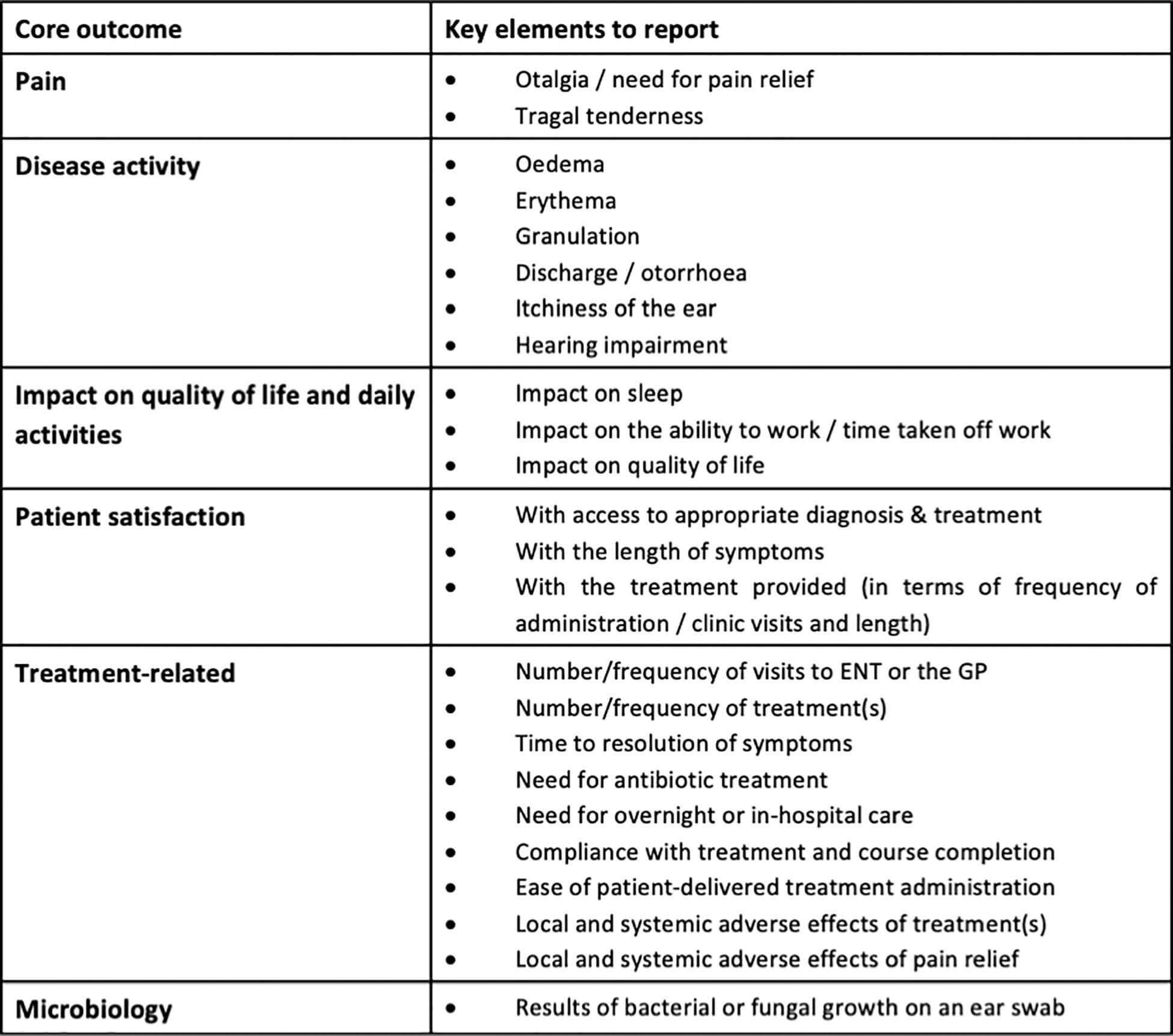
Figure 2. Core outcome set for acute otitis externa [2].
Outcomes of the project
This project has defined key parameters required for future research into AOE, outlining details for both the disease cohort and the outcomes. The involvement of former patients led to the most significant differences between our proposed COS and the outcomes reported in previous work. Many items considered important by patients were not mirrored in those previously adopted in the literature, such as effect on daily activities and quality of life. These outcomes may have been overlooked by researchers in a condition seen as relatively minor, of short duration and localised. The value of presenting the patient opinion to professionals could be seen in the Delphi responses, with former patients (but not clinicians) rating quality of life, effect on work and treatment satisfaction highly in the first round, and professionals then changing their responses to support inclusion of these in the second round. It has been noted that compliance with treatment for AOE is often poor and the reasons for this have not yet been explored [4]. The COS has the potential to identify interventions that patients find unappealing or difficult, which is important when considering treatment compliance on transfer to routine clinical practice.
“Many items considered important by patients were not mirrored in those previously adopted in the literature, such as effect on daily activities and quality of life”
Involvement of former patients throughout the project has highlighted areas where current clinical practice may be improved, specifically in the delay of appropriate management and the control of pain. Patient-derived priorities for research also did not fully align with those typically addressed in existing work. While most research to date has focused on resolution of infection and inflammation, improving symptom control and the ease of use and tolerability of treatments were important to patients. This reinforces the increasingly acknowledged role of patient and public involvement in research agenda setting and study design.
The INTEGRATE team and multidisciplinary collaborators behind this project hope that the development and adoption of consensus diagnostic criteria and COS will help to standardise future research in AOE. Developing a COS is only the first step towards determining how we should measure the effectiveness of an intervention, defining the type of outcome important to stakeholders, but not the way in which they are measured [1]. For this reason, work with patients is currently underway to develop a patient-reported outcome measure for AOE.
References
1. Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: version 1.0. Trials 2017;18(Suppl 3):280.
2. Smith ME, Hardman JC, Mehta N, et al. Acute otitis externa: Consensus definition, diagnostic criteria and core outcome set development. PLoS ONE 2021;16(5): e0251395.
3. Dalkey NC. The Delphi Method: An Experimental Study of Group Opinion. Santa Monica, CA; RAND Corporation; 1969.
4. Kaushik V, Malik T, Saeed SR. Interventions for acute otitis externa. Cochrane Database Syst Rev 2010;(1):CD004740.