Eliot Shearer shares the progress being made with newborn hearing screening 60 years on from where it started, and future directions for identifying hearing loss using physiologic, genetic and cCMV screening.
Newborn screening had its birth in the early 1960s, when Guthrie developed a bacterial inhibition assay to quickly and easily screen for the metabolic disease phenylketonuria using a small amount of blood obtained from heel-sticks of newborns [1].
This discovery led to the development of newborn screening worldwide. The goal of newborn screening is to provide presymptomatic and rapid diagnosis for treatable disorders for which an early intervention is critical, for example congenital hypothyroidism and phenylketonuria. Today, nine of these disorders are evaluated by the newborn screening programme in the UK while, in the US, screening varies by state but can include as many as 66 disorders. These programmes have been enormously successful in identifying treatable disorders in young children.
In the early 2000s, after mounting evidence detailing the importance of early detection and treatment for childhood hearing loss [2] universal newborn hearing screening (NBHS) was incorporating into newborn screening programmes in the US and the UK. The goal of universal NBHS is to identify children with significant, permanent, hearing loss. Although screening approach varies by region, current NBHS methods are physiologic – they test the response of cochlear hair cells to sound (otoacoustic emissions (OAE)) or the response of neural pathway to sound (automated auditory brainstem response (AABR)).
“Universal NBHS has been widely adopted, with more than 98% of newborns in the UK and US undergoing screening according to the most recent data”
Universal NBHS has been widely adopted, with more than 98% of newborns in the UK and US undergoing screening according to the most recent data. Universal NBHS has been enormously successful at early identification of children with hearing loss, increasing the number of children identified in the US per year from 855 in the year 2000 to 6432 in 2018 (750% increase) [3]. In the US, data from more than 11.7 million newborns in the years 2015-2017 showed that hearing loss was identified in 16.51/10,000 births, which far exceeded the next most common diagnosis, primary congenital hypothyroidism, identified in 6.00/10,000 births [4]. In fact, hearing loss was the primary condition identified by newborn screening, comprising 50% of all conditions identified by the newborn screening programme [4]. This earlier identification of children with hearing loss has led to improved speech and language outcomes [5].
Clearly, the current physiologic NBHS is enormously important and has been successful at achieving the goal of identifying children with permanent hearing loss. In addition, as a screening test, OAE and AABR are relatively low cost, provide an immediate answer, are relatively easy to administer, and are highly sensitive for moderate-to-severe hearing loss. However, there are ways by which the physiologic NBHS could be improved. As currently implemented, the physiologic NBHS may not identify children with specific types of hearing loss: those with mild-moderate deafness, those with deafness outside the newborn period, and children with auditory neuropathy. In addition, there is no aetiologic information provided at screening, and there is high loss-to-follow-up rate (25.9% for most recent data in the US) [3]. So while the current universal NBHS has been remarkably successful, there is room for improvement.
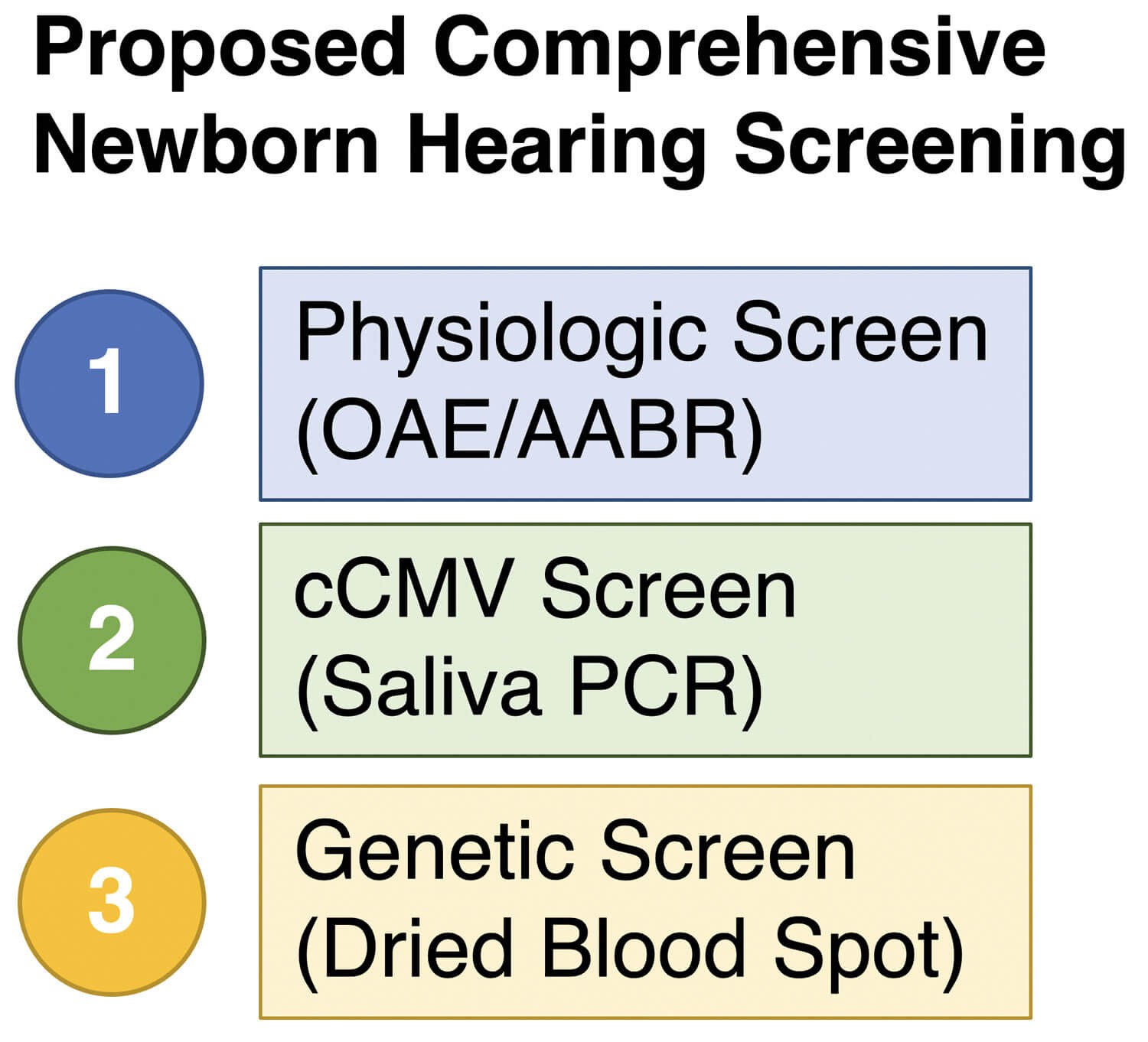
Proposed comprehensive newborn hearing screening. This would include: (1) physiologic screen, as performed currently with otoacoustic emissions (OAE) or automated auditory brainstem response (AABR); (2) congenital cytomegalovirus (cCMV) screen from saliva; and (3) genetic screen from dried blood spot. As detailed in the article, the combination of these three approaches would increase the number of children identified with permanent hearing loss, among other benefits.
In order to improve the current physiologic NBHS, an understanding of the causes of newborn hearing loss is integral. In developed countries, approximately 50% of newborn hearing loss is due to a genetic cause, approximately 30% due to structural cochleovestibular abnormalities, and approximately 20% is due to environmental causes, the most common of which is congenital cytomegalovirus (cCMV) [6]. Hearing loss due to cCMV is often mild, or unilateral, two types of hearing loss that are more difficult to detect using the current physiologic newborn hearing screen. In addition, because the cytomegalovirus is ubiquitous in our environment, testing must be performed before three weeks of age to accurately diagnose cCMV hearing loss. It is for these reasons that many have recommended incorporation of universal cCMV screening from saliva. In several U.S. states, cCMV screening is performed after failed NBHS and universal cCMV is advocated.
To date, 121 genes and thousands of mutations have been identified that cause non-syndromic hearing loss (https://hereditaryhearingloss.org). This extreme genetic heterogeneity makes genetic testing for hearing loss difficult. In addition, the most common causes of genetic hearing loss vary significantly by ethnic background [7]. Fortunately, the past 15 years have seen the advent of new DNA sequencing technologies that have allowed comprehensive genetic testing for hearing loss. Comprehensive genetic testing is a method by which all known hearing loss genes are sequenced simultaneously. This method was first described in 2010 [8] and quickly became the standard of care for genetic testing for hearing loss [9]. Comprehensive genetic testing for hearing loss provides a diagnosis in approximately 50% of children with congenital profound sensorineural hearing loss [7]. However, this type of testing is expensive to adapt to a screening platform. A more limited genetic screen of a handful of the most common hearing loss mutations is a more viable, and less costly approach.
“We recommended moving towards a comprehensive genetic screen that incorporates physiologic, cCMV, and genetic screening”
In 2019, a working group was convened to address the possibility of improving the current physiologic newborn hearing screen using genetics. We recommended moving towards a comprehensive genetic screen that incorporates physiologic, cCMV, and genetic screening [10]. Several recent studies have provided evidence that at least a limited genetic screen may improve the current universal NBHS. In one study, 1,172,504 Chinese newborns underwent NBHS that incorporated a physiologic screen as well as a genetic screen of 20 of the most common causes of genetic hearing loss in the Chinese population [11]. Of these, 360 newborns (0.3%) had a positive genetic screen (hearing loss mutations identified) and 42% of newborns who had a positive genetic screen had passed their NBHS and would have been missed. Therefore, incorporating genetic screening improved the ability to detect children with hearing loss. Further research is needed to validate these outcomes in an ethnically heterogeneous group and by incorporating cCMV screening. Genetic screening could identify hearing loss that is more mild, rapidly progressive outside of the newborn period, and would provide aetiologic information.
“Now, 60 years after Guthrie developed the first newborn screening method, it is time to take the next step in NBHS”
Widespread adoption of universal NBHS has been successful at its primary goal of identifying more children with hearing loss. Now, 60 years after Guthrie developed the first newborn screening method, it is time to take the next step in NBHS. A comprehensive NBHS that incorporates physiologic, genetic, and cCMV screening would address several weaknesses of the current, physiologic-only screen. In addition, aetiologic information would be provided sooner, which could lead to decreased time to diagnosis and habilitation, if required. Further research is needed as to the most cost-effective genetic screening method to be used in an ethnically heterogeneous population like the US and UK.
References
1. Guthrie R, Susi A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics 1963;32:338-43.
2. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of Early- and Later-identified Children With Hearing Loss. Pediatrics 1998;102(5):1161-71.
3. Centers for Disease Control and Prevention. 2018 Annual Data Early Hearing Detection and Intervention (EHDI) Program.
www.cdc.gov/ncbddd/hearingloss/ehdi-data.html
Last accessed May 2021.
4. Sontag MK, Yusuf C, Grosse SD, et al. Infants with Congenital Disorders Identified Through Newborn Screening — United States, 2015–2017. Morbidity Mortal Wkly Rep 2020;69(36):1265-8.
5. Yoshinaga-Itano C, Sedey AL, Wiggin M, Mason CA. Language Outcomes Improved Through Early Hearing Detection and Earlier Cochlear Implantation. Otol Neurotol 2018;39(10):1256-63.
6. Lieu JEC, Kenna M, Anne S, Davidson L. Hearing Loss in Children. Jama 2020;324(21):2195-205.
7. Sloan-Heggen CM, Bierer AO, Shearer AE, et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum Genet 2016;135(4):441-50.
8. Shearer AE, DeLuca AP, Hildebrand MS, et al. Comprehensive genetic testing for hereditary hearing loss using massively parallel sequencing. Proc National Acad Sci 2010;107(49):21104-9.
9. Shearer AE, Smith RJH. Massively Parallel Sequencing for Genetic Diagnosis of Hearing Loss: The New Standard of Care. Otolaryngology Head Neck Surg 2015;153(2):175-82.
10. Shearer AE, Shen J, Amr S, et al. A proposal for comprehensive newborn hearing screening to improve identification of deaf and hard-of-hearing children. Genet Med 2019;21(11):2614-30.
11. Wang Q, Xiang J, Sun J, et al. Nationwide population genetic screening improves outcomes of newborn screening for hearing loss in China. Genet Med 2019;21(10):2231-8.