Dave was a named author on nearly 50 peer-reviewed articles on vestibular schwannoma. His PhD was on physiological mechanisms of tinnitus in vestibular schwannoma. Here, Prof Saeed describes his current approach to the management of such tumours.
Vestibular schwannoma is the commonest tumour of the cerebellopontine angle (80%) and accounts for around 8% of all intracranial tumours. The commonest primary presenting symptoms are audio vestibular. Hearing health professionals are often the first contact for patients with potential symptoms of vestibular schwannoma, with the majority then being seen and diagnosed by otorhinolaryngologists.
Management
The subsequent management of the patient has evolved over the last 20 years and is determined by a number of factors which can broadly be divided into two domains: patient factors and clinical team factors. Patient determinants include the age and general health of the patient, size of tumour at presentation and the clinical assessment such as hearing, balance, cranial neuropathies and other neurological symptoms and signs. Clinical team factors include accrued experience of what should comprise a combined neuro-otology/neurosurgical/radiology/radiation oncology and allied specialties team. This team should have access to all treatment modalities, preferably within their healthcare organisation – this, in the opinion of the author, represents the benchmark standard.
Finally, historic legacies, geographic location and emotional investment in surgical decision making continue to influence the management of such patients in the modern era, despite ample robust clinical and epidemiological evidence that should allow equipoise to be the norm at a national and international level.
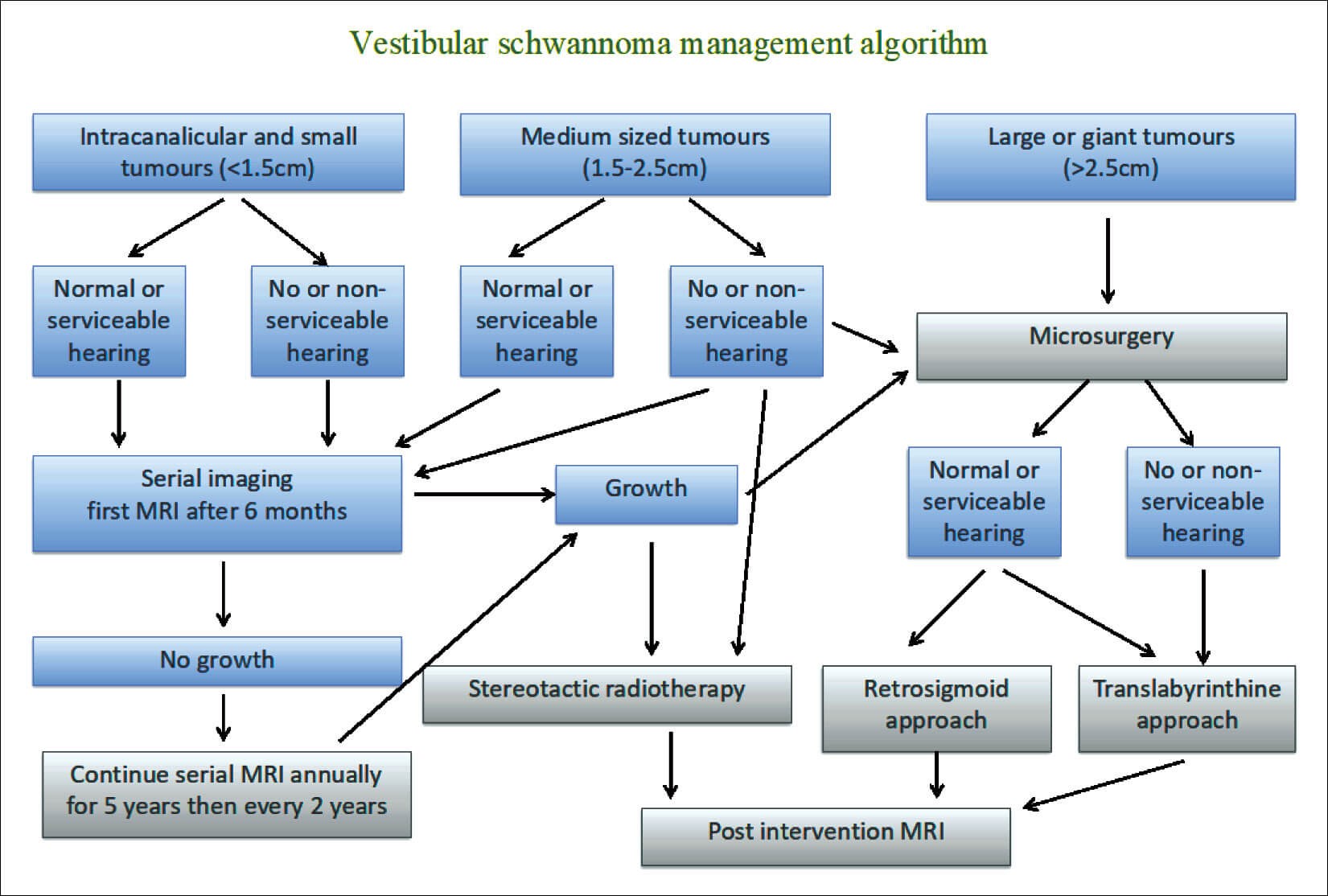
Figure 1.
Figure 1 is the author’s broad algorithm for managing the patient presenting with a diagnosis of vestibular schwannoma. The tumour size refers to the maximum diameter in the cerebellopontine angle (CPA) thereby excluding the component in the internal meatus.
"A critical part of the management is a comprehensive explanation of the diagnosis and our current understanding of the natural history of such tumours, with particular reference to their benign nature"
At initial diagnosis, a critical part of the management is a comprehensive explanation of the diagnosis and our current understanding of the natural history of such tumours, with particular reference to their benign nature (the author is yet to see a malignant unilateral sporadic tumour in 30 years of practice). In addition, the patients should be counselled that those tumours that do grow, do so slowly in the vast majority and, for small and medium sized tumours, there is little immediate threat to their life. Reference should however be made to the ongoing threat to the hearing in the affected ear and that in around 5%, the hearing can drop precipitously, presumably due to cochlear ischaemia rather than tumour growth. Appropriate auditory and tinnitus habilitation is offered, although hearing aids in a unilateral retrocochlear hearing loss may confer only limited benefit. Advice should be given about the impact on the patient’s balance and the role of vestibular rehabilitation, as well as advice about driving. Finally, in patients under the age of 35 years, full spinal MR imaging (MRI) is organised as part of the protocol to exclude neurofibromatosis type 2.
Small tumours
Intracanalicular or tumours less than 1.5cm in the CPA at diagnosis, irrespective of hearing, are initially managed through surveillance. This is predicated on not just the author’s experience of managing over 1000 referrals over three decades but also robust epidemiological data, particularly from Denmark and the large USA tumour registries that show that as many as 65% of small tumours do not grow over prolonged periods of observation. As per the British Skull Base Society guidelines the first MRI is six months after the diagnostic scan. This management plan is invariably well received by the patient - it is inappropriate to irradiate such tumours from the outset to control growth without establishing growth and it is even more inappropriate to subject a patient to major surgery without establishing growth as, even in experienced hands, surgery carries a small risk of significant complications and a substantial risk of complete hearing loss. If the six months MRI shows no radiological change in the tumour, then surveillance continues as per the author’s algorithm. If at any point the tumour grows, then intervention is instigated, informed by tumour size, residual hearing and patient preference.
SEE ALSO
As described above, this initial conservative approach is the mainstay of management of small tumours across the UK and in many parts of the world. However, there are centres internationally that adopt an alternative approach which is to undertake surgical removal of small tumours at diagnosis with attempted hearing preservation and to render the patient tumour free. Whilst this approach seems like an attractive option, there remains the risk of complications such as facial palsy and hearing preservation at the preoperative levels fails in 30-50%, even in highly experienced hands – the author therefore does not countenance this approach in his patients.
Medium-sized tumours
Patients presenting with a tumour between 1.5cm and 2.5cm represent the most challenging group in terms of initial management, as all three options are justifiably available to them: surveillance to establish further growth, stereotactic radiotherapy (fractionated, gamma knife, cyberknife) from the outset predicated on the fact that the tumour has clearly grown to reach this size, or surgical removal. Careful consideration is given to all the patient factors described above and a decision is reached with the patient.
Large and giant tumours
Once a tumour reaches 2.5cm in the CPA and certainly 3cm, the patient will invariably have additional neurological deficits including trigeminal symptoms and occasionally lower cranial nerve symptoms. Some will also present with signs of brainstem and cerebellar compression and hydrocephalus. The management of large tumours is, therefore, surgical removal by a combined ENT skull base and neurosurgical skull base team. Both disciplines bring specific skillsets to the surgery to the benefit of the patient, and the days of the sole specialty surgeon should be relegated to the dustbins of history.
"The key determinants of outcome are the size of the tumour and hearing at presentation and the depth and breadth of the team managing such patients"
There are potentially three surgical approaches to such tumours, although the middle fossa approach is only really applicable to intrameatal tumours or tumours with a very small CPA component. So, in reality, for large tumours there are two surgical approaches: translabyrinthine and retrosigmoid.
Translabyrinthine approach
This approach involves an extensive lateral petrosectomy undertaken by the skull base otologist and, contrary to received wisdom, unless the temporal bone is particularly small or poorly pneumatised, there is no upper limit of tumour size. The key determinant is the ability of the surgeon to undertake an adequate petrosectomy with skeletonisation of the internal auditory meatus (IAM) through 270 degrees from the fundus to the porus as well as the posterior and middle fossa dura from the jugular bulb to superior petrosal sinus. Once the posterior fossa dura is opened, the tumour resection should be a combined effort to maximise tumour resection with preservation of vital structures based on the experience and expertise of the surgeons involved. The main advantages of this approach include early identification of the facial nerve in the fundus of the IAM, a direct trajectory to the tumour and no retraction of the cerebellum once CSF has been drained from the basal cisterns. The main disadvantages, aside from the complete loss of hearing, include occasional limited access to the inferior CPA due to the jugular bulb (which can be skeletonised and decompressed for access) and the need to harvest abdominal fat to fill the petrosal cavity at the end of the procedure.
Retrosigmoid approach
This is the classic neurosurgical approach to the posterior fossa and gives a panoramic view of the CPA. It also has the advantage of potential hearing preservation surgery. However, skeletonisation and visualisation of the fundus of the IAM is hampered by the posterior semi-circular canal and, as such, identification of the facial nerve at the fundus is less straightforward. Endoscopes may help in this regard. In addition, a degree of cerebellar retraction is invariably required which may have postoperative consequences. Nevertheless, the retrosigmoid approach is a vital part of the skull base team’s armamentarium and, based on patient factors described above, there is an even split between the retrosigmoid and translabyrinthine approaches in the author’s unit.
The paradigm shift
Over the last 20 years, many skull base units, including that of the author have changed their management of patients presenting with large tumours from total removal with potential harm to key cranial nerves and the brain, to near-total (95%), sub-total (90%) or partial (more than 80%) tumour removal with enhanced preservation of facial function. The residual tumour is then serially imaged and stereotactic radiotherapy utilised if the remnant grows – often the biology of the residual tumour changes and further intervention is not required. From the patient’s perspective normal or near-normal facial function (House-Brackman grades 1 and 2) is a good outcome - anything worse than that has an impact on the patients for the rest of their life. Postoperative facial function is therefore a good marker of how well the surgery has gone as, invariably, if the facial function is good, so is the function of the lower cranial nerves and brainstem. Figure 2 illustrates the impact of less than total removal of large tumours on preserving facial function in the author’s last 95 consecutive cases.
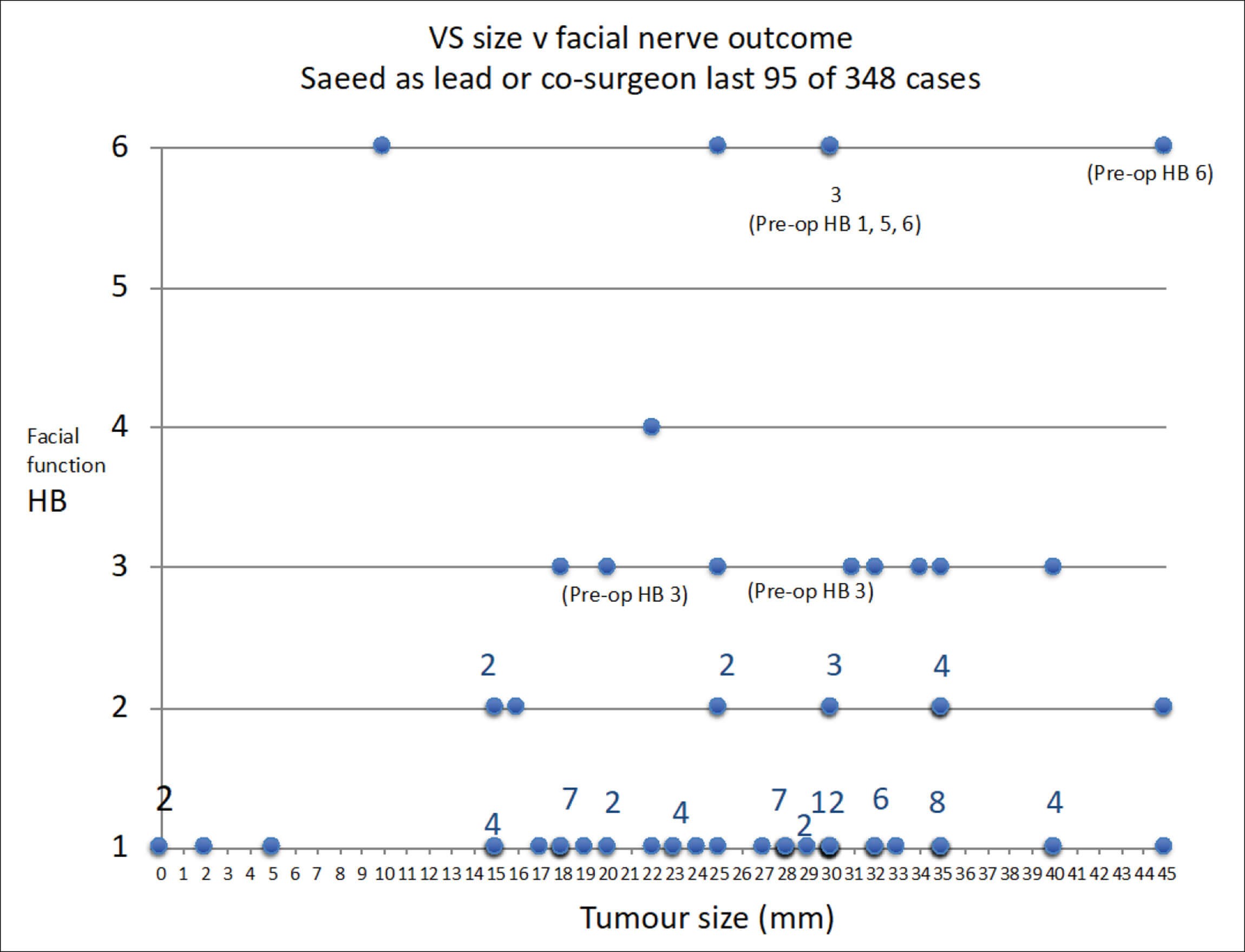
Figure 2.
Summary
The management of a patient presenting with a unilateral sporadic vestibular schwannoma has evolved over the last two decades in terms of management options, timing and nature of intervention and extent of surgical resection in those undergoing surgery for large tumours. The key determinants of outcome are the size of the tumour and hearing at presentation and the depth and breadth of the team managing such patients.
Further reading
1. Shapey J, Bradford R, Saeed SR. Surveillance management of vestibular schwannoma: present and future strategies. Vestibular Schwannomas. In: Handbook of Clinical Neurology. Elsevier; 2021.
2. Saeed SR, Hall AC. Vestibular Schwannoma. In Roland N, McRae D, McCombe AW (Eds.). Key topics in Otolaryngology – Head and Neck Surgery, 3rd Edition. Thieme; 2019.
3. Saeed SR, Skilbeck C. Surgical Management of Vestibular Schwannoma. In Watkinson J, Clarke R (Eds.). Scott-Brown’s Otorhinolaryngology, Head & Neck Surgery 8th Edition; 2018.
4. Saeed SR. Vestibular schwannoma/Cerebellopontine angle tumours. In Sethi N, Pearson A, Bajaj Y (Eds.). Key Clinical Topics in Otolaryngology. Japyee UK; 2016.
5. Skillbeck C, Saeed SR. Cerebellopontine angle tumours. In Hussain SM (Ed.). Logan Turner’s Diseases of the Nose, Throat and Ear, 11th ed. London, UK; CRC Press; 2015.