It’s time to improve and personalise care for adults with ANSD. Knowing the lesion site could open up treatment opportunities like cochlear implants or new cell therapies.
Auditory neuropathy spectrum disorder (ANSD) comprises a group of hearing disorders characterised by abnormal auditory nerve and / or inner hair cell (IHC) function despite preserved outer hair cell function. This leads to relatively preserved audiometric thresholds, but disproportionate difficulties with speech perception.
ANSD is often viewed as a predominantly paediatric condition, partly due to better defined clinical pathways, facilitating diagnosis compared with adults. However, ANSD can persist into adulthood or develop later in life. Recent studies estimate that around 5% of adults with mild to moderate sensorineural hearing loss may also have undiagnosed and unaddressed ANSD [1]. True prevalence is likely significantly underestimated, given inconsistent diagnostic practices and limited treatment options.
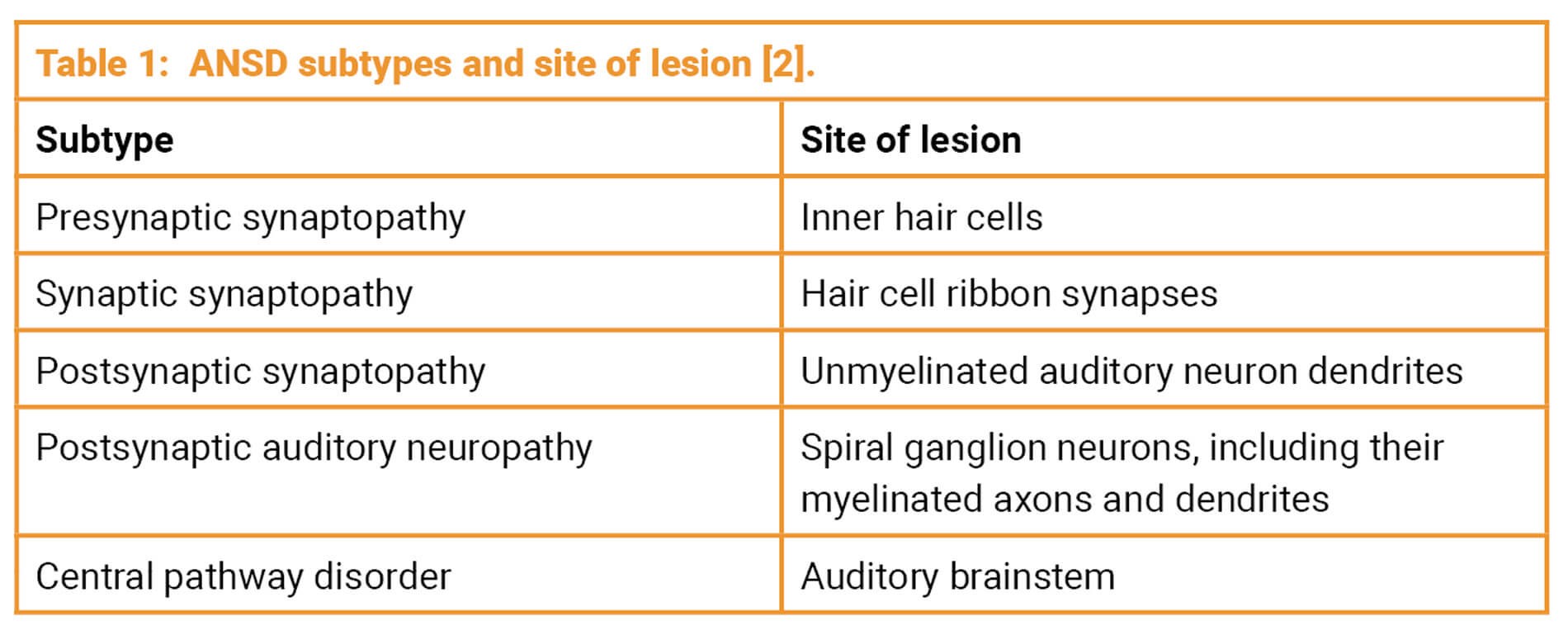
Accurate identification of the ANSD subtype (see Table 1) is essential for selecting appropriate treatment and anticipating prognosis, and can be achieved through a combination of audiological, electrophysiological and, where appropriate, genetic or imaging assessments.
Challenges in diagnosing ANSD in adults
Many adults with ANSD do not receive a formal diagnosis, often due to the absence of a comprehensive and appropriate test battery. The reliance on pure tone audiometry (PTA) – without routine speech or objective testing – limits the ability to identify, or even suspect, ANSD in adults. Notably, around 10% of patients seen in audiology services report hearing difficulties despite normal PTA thresholds [3]. Adults with ANSD may also present with a measurable, aidable sensory loss, yet continue to experience disproportionately poor speech perception. These individuals are frequently offered repeated hearing aid adjustments or rehabilitative strategies (e.g. listening tactics), rather than being offered further diagnostic evaluation.
ANSD detection rates could be significantly improved through wider access to advanced speech and electrophysiological testing – such as speech audiometry, speech-in-noise testing, electrocochleography (including cochlear microphonics) and auditory brainstem response (ABR) testing. Although genetic testing is a valuable diagnostic tool, it remains underutilised, with referral rates from ENT departments remaining low [4]; similarly, imaging can play an important role in identifying structural or neural abnormalities but is not routinely employed.
"The reliance on pure tone audiometry (PTA) – without routine speech or objective testing – limits the ability to identify, or even suspect, ANSD in adults"
Clinicians with access to genetic testing services can make more informed decisions regarding treatment options, as highlighted by Matthew Smith, Honorary Consultant Otologist at Cambridge University Hospitals: “We have been performing NHS-funded genetic testing on increasing numbers of children and adults with hearing loss, and this is now routine for most of our cochlear implant patients. Combined with ABR and other tests, this has allowed us to better target existing and emerging novel therapies to the patients who will benefit the most, including adults with ANSD.”
Cochlear implants for ANSD patients
Cochlear implants (CIs) can be beneficial for individuals with ANSD, especially those with auditory synaptopathies impacting the IHCs and the ribbon synapses [5]. However, professional engagement conducted by Rinri Therapeutics with UK hearing loss clinicians reveals a significant paucity of CI referrals for adults with ANSD. Despite eligibility under UK CI funding guidelines, adults with ANSD are rarely referred. Discussions with clinicians from audiology and CI centres across the UK suggest two main reasons: a lack of awareness that adults with ANSD qualify for CI and a misconception that ANSD predominately affects children. This shortfall in eligible referrals may be limiting access to CIs for ANSD patients who could otherwise benefit from this intervention.
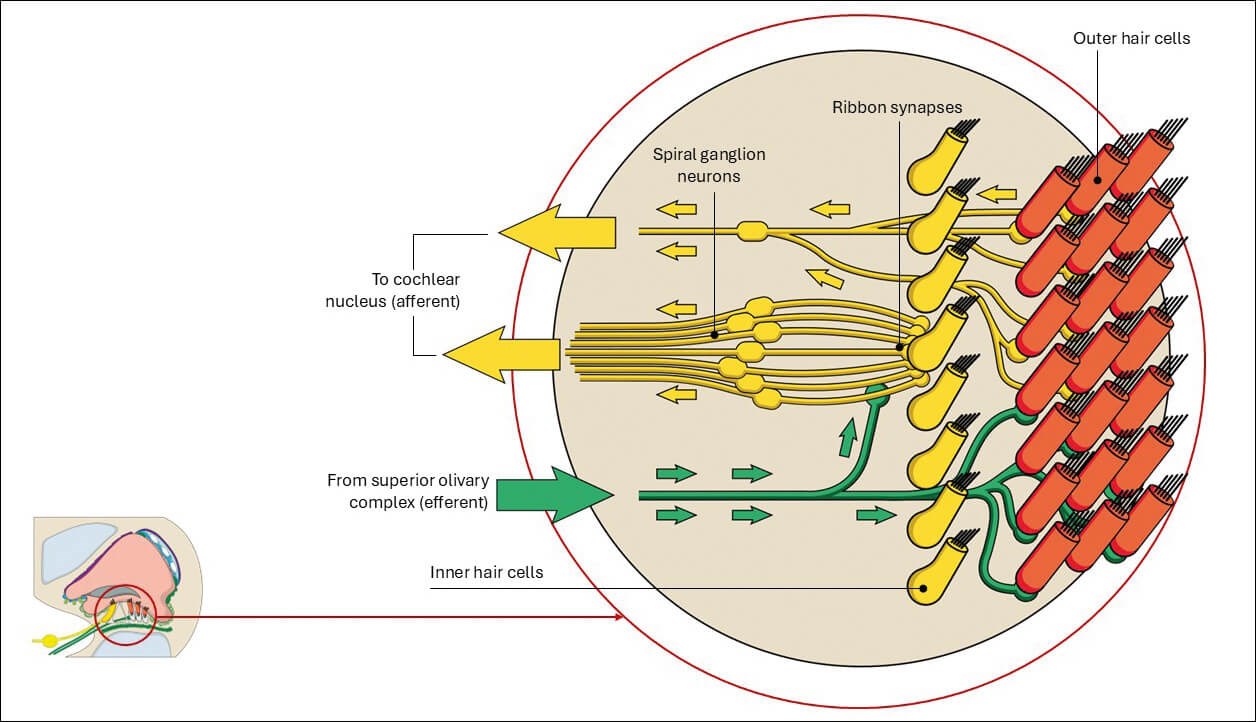
Figure 1: Innervation of the cochlea.
Clinicians more experienced in adult ANSD referrals and cochlear implantation report positive experiences and favourable outcomes in this population. For example, Peter Monksfield, Consultant ENT Surgeon at University Hospital Birmingham, highlights that: “Patients with auditory neuropathy generally do well with a cochlear implant. We have published a systematic review looking at outcomes following cochlear implantation for this group of patients and found that the hearing benefit was modest to significant in the vast majority of cases [5]. We are encouraged by this data and would recommend a referral to consider a cochlear implant for these patients.”
Another barrier to CI referral for adults with ANSD is the challenge of establishing a formal diagnosis. Access to advanced audiological and genetic diagnostic services is often limited and, as such, clinicians should refer suspected cases to a CI centre or specialised clinic for comprehensive evaluation and management. This sentiment is echoed by Andrew Soulby, Principal Clinical Scientist and Adult Hearing Implant Coordinator at St Thomas’ Hearing Implant Centre: “As a hearing implant centre, we currently receive a low volume of referrals to consider CIs for adults diagnosed with ANSD. Counselling these individuals is always challenging as our experience shows us that these patients can derive benefit from CIs but that the degree of this benefit can be very difficult to predict beforehand. More robust referral routes to implant centres and incorporating genetic testing to determine the origin of the ANSD into the standard of care would undoubtedly be beneficial for this patient group”.
The author, Douglas Hartley, Chief Medical Officer at Rinri Therapeutics and Consultant ENT Surgeon at Nottingham University Hospitals NHS Trust, also stresses the importance of genetic testing for adults with ANSD. In his ENT practice, genetic testing is increasingly valuable in assessing adults with suspected ANSD. It can help distinguish between presynaptic and postsynaptic subtypes, guiding both prognosis and treatment decisions. Identifying causative variants can support earlier and more confident referral for cochlear implantation or, alternatively, indicate when a patient is less likely to benefit from a CI, ensuring therapy is matched to pathology more precisely.
Emerging therapies for neural hearing loss
For patients with post-synaptic auditory neuropathy, CIs – whilst considered the gold standard for treating sensorineural hearing loss – are often limited in the benefit they provide, as they rely on the presence of functional auditory neurons [2]. In such cases, advanced therapy medicinal products (ATMPs), including cell and gene therapies, may offer a more targeted approach by addressing the underlying pathology of ANSD. These emerging therapies are being developed either as standalone treatments that could restore hearing without the need for devices, or as adjuncts to enhance the effectiveness of hearing aids or CIs.
For patients with neural hearing loss – including individuals with post-synaptic auditory neuropathy – a cell therapy specifically targeting auditory neuron loss and dysfunction could offer substantial benefit. This unmet clinical need is currently being explored by Rinri Therapeutics, who are developing Rincell-1, a first-in-class cell therapy aimed at regenerating auditory neurons and restoring neural function. By addressing the underlying neural deficit, this approach has the potential to enable patients to derive greater benefit from cochlear implantation.
A UK-based first-in-human clinical trial is planned to begin shortly, evaluating the safety and efficacy of Rincell-1. The study will recruit adults with either post-synaptic auditory neuropathy or presbycusis who are undergoing CI surgery. For both groups, this trial marks a crucial step towards developing biological treatments that address the underlying cause of hearing loss. Furthermore, it offers a rare yet valuable opportunity to re-evaluate current pathways for diagnosing ANSD in adults and to strengthen referral practices.
Conclusions
Undoubtedly, ANSD remains a complex and often overlooked contributor to hearing loss in adults. There is growing recognition that CIs can be an effective treatment option for adults with ANSD. To support better outcomes, clinicians need to look beyond the standard audiogram and incorporate a wider range of diagnostic tools. When combined with genetic profiling, these approaches help guide more informed, individualised decisions about care. Finally, sharing real-world outcomes – through both publication of clinical data and direct feedback to referring clinicians – will be critical to advancing diagnostic and management strategies, for patients with ANSD who receive CIs. Emerging therapies offer new hope for those with historically limited options. However, identifying and accurately diagnosing adult ANSD remains the first essential step.
References
1. Kim Y, Han JJ, Oh J, et al. Audiogram Configuration, Molecular Etiology, and Outcome of Cochlear Implantation in Postlingual Auditory Neuropathy Spectrum Disorder. Otol Neurotol 2023;44(7):471–8.
2. Shearer AE, Hansen MR. Auditory synaptopathy, auditory neuropathy, and cochlear implantation. Laryngoscope Investig Otolaryngol 2019;4(4):429–40.
3. Tremblay KL, Pinto A, Fischer ME, et al. Self-Reported Hearing Difficulties Among Adults With Normal Audiograms: The Beaver Dam Offspring Study. Ear Hear 2015;36(6):290–9.
4. Muzaffar J. Exploring the iceberg of adult cochlear implant assessment referrals: A multicentre national study [conference presentation]. ENTUK British Society of Otology Annual Meeting, Birmingham, UK. 2025, April 2-3.
5. Chaudhry D, Chaudhry A, Muzaffar J, et al. Cochlear Implantation Outcomes in Post Synaptic Auditory Neuropathies: A Systematic Review and Narrative Synthesis. J Int Adv Otol 2020;16(3):411–31.
Declaration of competing interests: DH and FM are employed as Chief Medical Officer and Clinical Manager respectively at Rinri Therapeutics.












