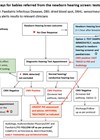
Why 1-3-6 and 9? They are arguably crucial intervention stages. Here, new science is explored which weaves together the threads of early intervention.
Imagine a newborn gazing at their caregiver’s face, hearing their voice and feeling the rhythm of their words. Within weeks, the baby begins to babble – an intricate dance of sound, sight and movement that lays the foundation of language. But, for a baby born deaf, that dance is disrupted. The rhythm falters. Brain development risks falling out of step.
That is the reality for 32 million children worldwide. Hearing aids and cochlear implants (CIs) can restore access to sound, but timing is everything. The central finding of our recent paper in Hearing Research is that sound must be restored within this window if language and cognition are to flourish [1]. What is new here is that the motor system, long treated as peripheral to language, is in fact central. When hearing infants babble, imitate and take conversational turns, they activate a motor-hearing-language loop that scaffolds phonology, vocabulary and executive function (Figure 1). When auditory access is delayed, that loop developmentally desynchronises. The brain then leans on visual and motor alternatives but the core circuits for hearing and language fall out of phase even before the first words emerge.

Figure 1: Simplified motor-hearing-language brain loop. The prefrontal cortex (magenta) supports executive function that initiate motor action (magenta arrow) in the premotor and motor cortex (in grey) that causes a motor act. This affects sensory input and, at the same time, provides top-down information about movement to the sensory areas (black arrows). These receive information on the change in the environment caused by the movement (auditory: bluish/purple; visual: orange; somatosensory: green) that is fed back to cognition (frontal cortex, dashed arrows), together forming a complex closed loop. Multisensory and language areas omitted for simplification.
By the time late auditory access arrives, motor routines have been practising without sound. The timing that normally binds perception to action is misaligned. That desynchronisation is the seed of the cognition gap many deaf children face. The practical implication is clear: restore access to sound before the age of nine months, a critical communication milestone. Deaf children are then positioned to develop language and thinking skills much closer to their hearing peers. This is first and foremost a brain-development story and, in close second place, a hearing-technology story.

Figure 2: A) Data from cochlear implant companies derived from sales data across a range of countries. Of children who receive their cochlear implant in the first three years of life, less than 20% do so within their first year. B) Analysing data in the years 2017 to 2023, shows no significant change in the percentage of children receiving cochlear implants in the first year of life.
The urgency is obvious. Newborn screening has advanced enormously, yet clinical pathways often drift into a ‘by-age-three’ mindset, especially for CI candidates (Figure 2). Our synthesis shows why that timeline misses the brain’s natural coupling window, and how it can be corrected.
Diagnostic confirmation, timely hearing-aid fitting and – in suitable cases – cochlear implantation under six months are feasible in experienced centres. Pairing these with parent-led multimodal communication ensures that turn-taking, gaze, vocal reciprocity and rhythmic games are dense and responsive from the very beginning. The equity dimension is stark: children most likely to face delays are in settings with limited resources, poor access to specialty care or complex referral pathways. The science gives a blueprint for closing a damaging gap.
This is advocacy based on the best available scientific evidence. We are mindful about safety profiles, risk–benefit judgments and the importance of family priorities. Not every infant is a candidate for implantation, and consent in infancy demands careful consideration. Family language goals matter, including bilingual approaches and respect for Deaf culture. Our emphasis is on measurable interaction quality as turn density and language-environment metrics have been shown to correlate with cortical development and executive outcomes. These are feasible to track and improve. Precision timing plus enriched interaction are key.
The lived experience mirrors the science. The best outcomes generally emerge when families experience timely referral from newborn screen to diagnosis, through hearing-aid fitting and, when appropriate, early implantation. Parents are then coached in responsive turn-taking, gestures and infant-directed speech. Thus, their babies engage in the very motor–hearing-language loops that imaging studies show are essential. Outcomes are best when interventions are aligned as close as possible with the brain’s developmental timetable. In clinical practice, this would follow a 1-3-6-9 monthly paradigm: 1 screening, 3 diagnosis, 6 access to sound (hearing aid trial etc) and 9 CI.
What distinguishes this work from earlier literature is the emphasis on timely integration. Prior research has documented auditory-cortical plasticity, while other studies highlighted the benefits of responsive caregiving. Here, we bring the threads together: functional MRI patterns showing motor involvement in early language, aligned with real-world measures of caregiver–infant interaction, and mapped against clinical timelines for hearing restoration. This triangulation yields a practice-changing claim: keep perception and action in step during the first year of life, and downstream gains in language and cognition are larger and more reliable. The key to optimising outcome is not simply more input, but synchronised, contingent interaction. For deaf infants, synchronisation requires timely access to sound and deliberate coaching of multimodal turns. Otherwise, plasticity is wasted. The take-home message is simple and profound: early access to sound, combined with enriched interaction, prevents desynchronisation and gives deaf infants their best chance to thrive.
The first nine months are not a waiting period – they are the crucible in which language, social connection and cognition are forged. For ENT specialists, audiologists and paediatricians, the message is urgent: do not delay sound access. With early intervention and family-centred support, deaf children can grow, learn and thrive, reaching their full potential alongside their hearing peers.
Reference
1. Kral A, Kishon-Rabin L, O’Donoghue GM, Romeo RR. Sensorimotor contingencies in congenital hearing loss. Hear Res 2025;467:109401.
Declaration of competing interests: None declared.