Surgery for nasal polyposis has evolved significantly in the last 30 years, and now the medical management may be on the cusp of a revolution.
Biologics using monoclonal antibodies to target specific immune pathways have introduced a paradigm shift in how we understand and treat patients with chronic rhinosinusitis with nasal polyps (CRSwNP). In 1984 the Nobel Prize in Physiology or Medicine was awarded to Niels Jerne, Georges Köhler and César Milstein for the discovery of how to create monoclonal antibodies.
Although biologic therapies in the past were developed largely to treat cancer and autoimmune disorders, these therapeutics have now entered our sphere and, as otolaryngologists treating patients with chronic rhinosinusitis, it is apparent that the need for education, research, and comparative outcomes research is lagging the approval of these advanced medications. It seems that suddenly our small world has opened to a completely new audience with a significant interest in treating this disorder that is not surgical, and this may cast uncertainty and apprehension in the minds of many practising otolaryngologists. Questions have arisen: will sinus surgery become obsolete? What is the cost to the health system, to the patient, to the practising otolaryngologist? What are the long-term side effects? How do we incorporate biologic therapies into our existing treatment algorithms?
“Although biologic therapies in the past were developed largely to treat cancer and autoimmune disorders, these therapeutics have now entered our sphere”
These questions and concerns are valid and, with the introduction of a new method of treating a disease, there is newfound opportunity. With this increased interest in our field, our patients will benefit tremendously but our field will benefit as well. Resilience, adaptability, and participation in the process of innovation is crucial. The importance of understanding the new data from phase 3 clinical trials and establishing fluency in the immunologic mechanisms underlying the disease is also paramount.
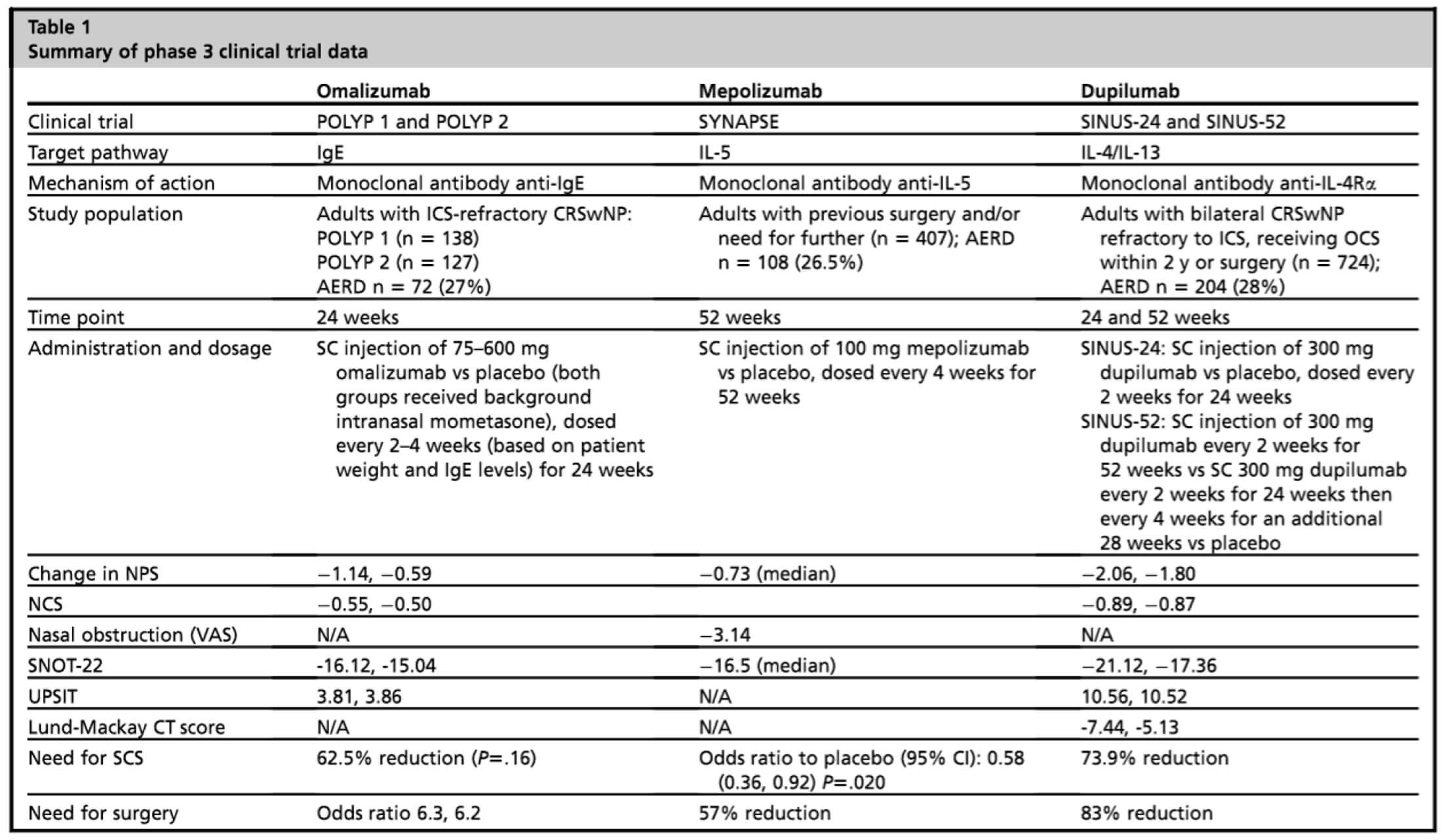
Originally published in Ramaswamy US Melder K, Patel VA, Lee SE. Current Evidence for Biologic Therapy in Chronic Rhinosinusitis with Nasal Polyposis. Otolaryngologic Clinics of North America. Volume 2021;54(4):689-99.
The majority of patients with CRSwNP appear to exhibit type 2 inflammation which is characterised by production of IL-4, IL-5, IL-13, activation of eosinophils and mast cells as well as isotype switching of B cells to produce IgE [1]. These patients also exhibit barrier disruption, mucociliary dysfunction, microbiome disturbances, goblet cell hyperplasia, and tissue remodelling. Clinically, this translates to symptoms of nasal obstruction, loss of smell, mucous overproduction, and facial pressure that significantly impacts the quality of life of our patients, which is often underestimated. Types 1, 2 and 3 inflammatory endotypes have been recognised in CRSwNP with respective Th1, Th2 and Th17-associated biosignatures.
A reliable biomarker which can help us counsel patients on prognosis and choose the best treatment strategy, however, remains to be determined. The combination of clinical factors with structured histopathology and available laboratory studies, such as a obtaining a complete blood count that includes an absolute eosinophil count and a serum total IgE level, may provide insight into understanding the patient endotype. It is also important to realise that one clinic visit may not be sufficient to fully understand the patient with CRSwNP. Caring for patients with a chronic illness such as CRSwNP involves understanding the symptoms that are most impactful and incorporating measurement of validated patient-reported outcome measures over time in conjunction with studying response to treatment utilising nasal endoscopy and culture data, if appropriate, to understand local microbiologic disturbances.
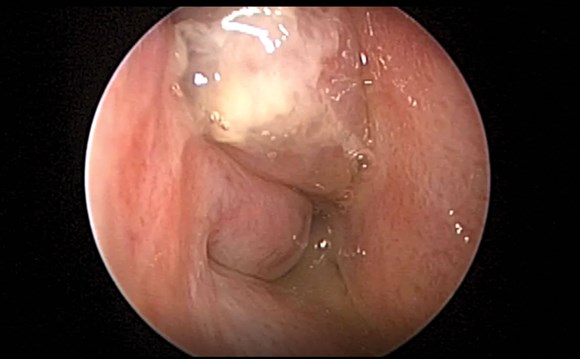
Click the image above to watch an endoscopic evaluation of a patient with CRSwNP.
Currently there are three biologics approved for treatment of uncontrolled CRSwNP in the US and the EU: dupilumab in 2019, omalizumab in 2020, and mepolizumab in 2021, all of which target different aspects of the type 2 inflammatory pathway. All three medications are humanised monoclonal antibody therapies. Dupilumab targets IL-4 receptor alpha which, in turn, inhibits both IL-4 and IL-13 signalling important in differentiation of CD4+ T-cells to Th2 cells. Omalizumab targets free IgE which is thought to play a central role in activation of mast cells, basophils, and eosinophils. Local polyclonal IgE production may also be involved in chronic inflammation. Mepolizumab targets free IL-5, which is important in eosinophil maturation and survival. Phase 3 clinical trials have shown efficacy of these therapeutics in the treatment of CRSwNP.
The LIBERTY SINUS-24 and SINUS-52 studies (dupilumab) [2], POLYP1 and POLYP2 (omalizumab) [3], and SYNAPSE (mepolizumab) [4]. Other studies of targeting IL-5 receptor alpha evaluating benralizumab (OSTRO) [5] have also been published and further studies targeting TSLP utilising tezepelumab are being conducted. With these trials, the importance of using a common language to measure outcomes in our patients has come to light. How do we measure the size of polyps? How is successful treatment measured? In these clinical trials the nasal polyp score has been utilised from a five-point scale (0-4 bilaterally) and validated symptom surveys such as the SNOT-22 and Visual Analogue Scale have been employed.
“With these trials, the importance of using a common language to measure outcomes in our patients has come to light”
Dupilumab was the first FDA-approved medication for patients with chronic rhinosinusitis. This is remarkable considering that otolaryngologists have been performing endoscopic sinus surgery for decades. With Walter Messerklinger’s landmark publication, Endoscopy of the Nose, in 1978 and the first endoscopic sinus surgical course at Johns Hopkins University in 1985, otolaryngologists have pressed onward to develop better techniques to manage sinus disease and there have been significant advancements in the understanding of how to perform sinus surgery safely, more effectively, and with excellent patient outcomes. In parallel with better surgical techniques, there have been advancements in more effective topical drug delivery strategies. Biologic therapies provide another option for our patients with uncontrolled CRSwNP. Moving beyond phenotypes, understanding the underlying pathophysiologic mechanisms of inflammation is important to best understand and select treatment for patients. This may involve a combination of strategies in a multifaceted patient-centred approach.
References
1. Kim JE, Jung K, Kim JA, et al. Engineering of anti-human interleukin-4 receptor alpha antibodies with potent antagonistic activity. Scientific Reports 2019;9:7772.
2. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019;394(1029):1638-50.
3. Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020;146(3):595-605.
4. Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021;9(10):1141-53.
5. Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J Allergy Clin Immunol 2022;149(4):1309-17.e12.
Declaration of competing interests: SL has received clinical trial funding and participated on advisory boards for AstraZeneca, Genentech, GSK, Sanofi Genzyme Regeneron.