A gastric inlet patch (GIP) is an island of heterotopic gastric mucosa found commonly in the proximal oesophagus just below the upper oesophageal sphincter. It is often underdiagnosed due to its location. Its importance and clinical relevance can be underestimated [1]. It is important to recognise as these patients can present to general practice, gastroenterology or ENT clinics with a wide variety of persistent throat symptoms and there are a number of treatment options available for a GIP.
Presentation
Many patients with a GIP are asymptomatic and it is an incidental finding during an upper gastrointestinal endoscopy. Symptoms associated with it include globus, cough, excessive throat clearing, hoarseness and dysphagia. This is thought to be due to excess acid or mucus production from the GIP. The most common associated condition that can cause this combination of symptoms is laryngopharyngeal reflux (LPR) [2]. A retrospective analysis of 870 patients with a GIP showed a significant association with symptoms of dysphagia, globus and upper respiratory symptoms [3].
Historically LPR was felt to be related to the reflux of gastric contents. The presence of acid-secreting cells in inlet patches challenges this notion. A number of studies have shown an increased incidence of LPR symptoms in patients with a GIP compared to those without [1].
Diagnosis
Studies have shown up to a 10-fold increase in the diagnosis of an inlet patch when there is a specific focus on that part of the oesophagus during endoscopic assessment with a targeted aim of demonstrating a GIP. This has been shown to be increased by up to three-fold with use of advanced endoscopic imaging, such as narrow band imaging. Incident rates vary between 0.1% and 10%. The quality of endoscopy is a likely contributory factor to this variability [2]. Clinicians may not report the presence of an inlet patch when identified due to an underestimating of the clinical relevance of this [1]. Therefore, the current incidence rates are likely an under-reporting of the ‘real world’ values.
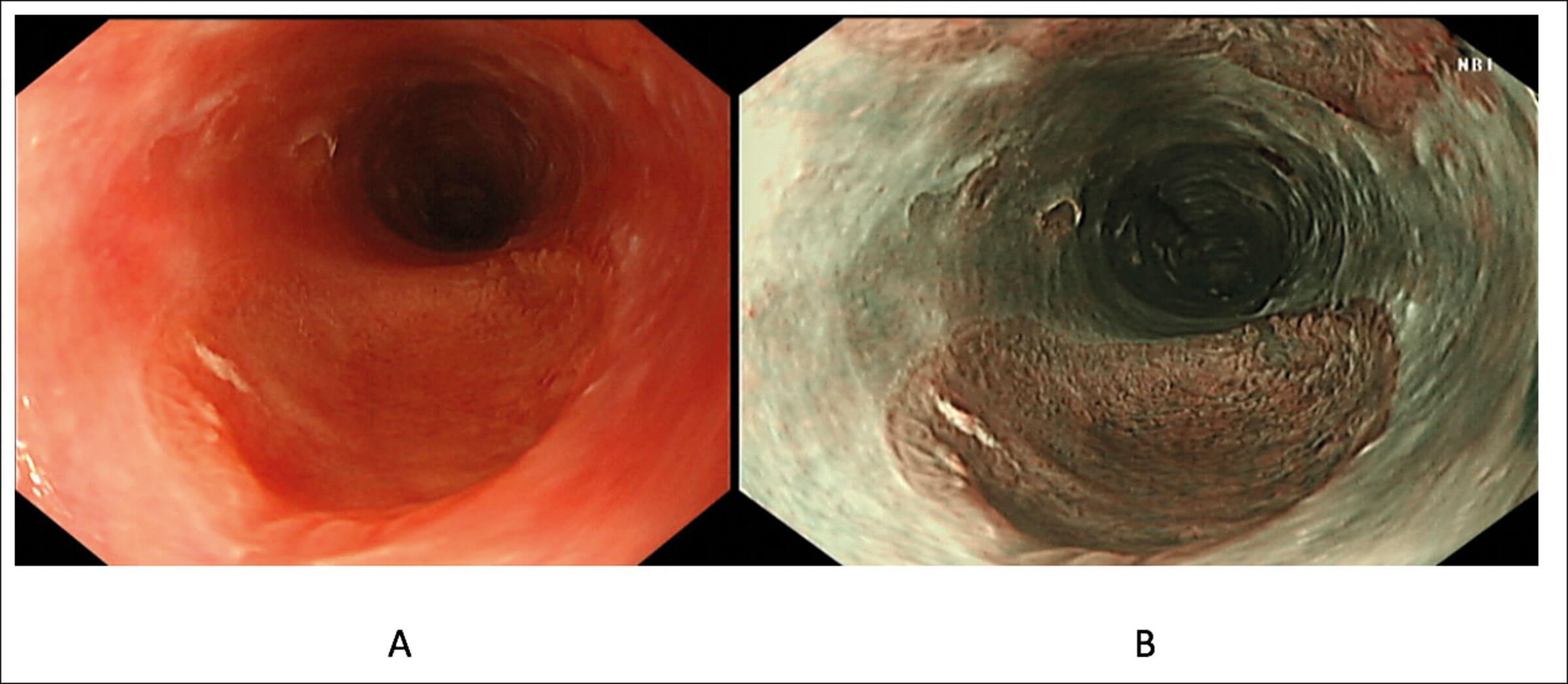
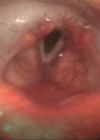
Figure 1. An inlet patch in the proximal oesophagus assessed in white light (A) and narrow band imaging (B).
An upper GI endoscopy is the gold standard for diagnosis of a GIP. It is usually identified 15-21cm from the mouth. It has the appearances of a salmon coloured and round/ovoid patch with clearly defined borders (Figure 1). It is best identified during slow withdrawal of the endoscope in the upper oesophagus with repeated short insufflations in combination with narrow-band imaging [2].
"Clinicians may not report the presence of an inlet patch when identified due to an underestimating of the clinical relevance of this"
Biopsies are required from an inlet patch if there is any evidence of mucosal irregularity, or a confirmation of a diagnosis is required prior to endoscopic treatment [1].
Barium swallows are often ordered as part of the diagnostic workup of patients with globus in the ENT clinic. This is not the recommended diagnostic modality for ruling out a GIP, however imaging findings associated with a GIP may include irregular outline, rim-like shadows and small indentations in the oesophageal wall [2].
Treatment options
Medical
Patients with no symptoms do not need treatment for a GIP. However, if patients are symptomatic then first-line medical treatment would be with a proton pump inhibitor (PPI), particularly as the majority of symptoms in these patients is secondary to acid production from the GIP [1].
Endoscopic
In patients not responsive to PPI treatment, endoscopic treatment is a potential option. In benign inlet patches, ablative treatment with argon plasma coagulation (APC) or radiofrequency ablation (RFA) has shown to be beneficial.
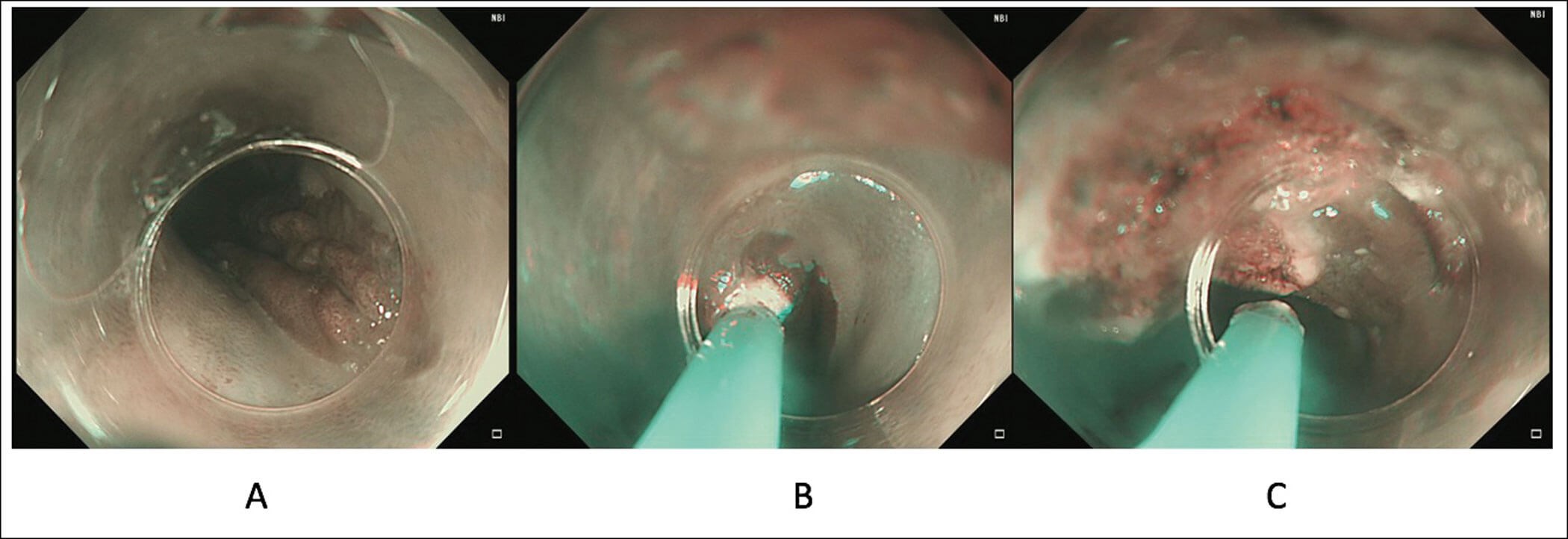
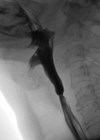
Figure 2. Treatment of a patient with large symptomatic gastric inlet patch (A) using APC where a cap
is used to provide stability in the proximal oesophagus and allow for targeted treatment (B and C).
Argon plasma coagulation
This has been shown to be an effective modality for a GIP. Using a cap is useful to provide stabilisation in the proximal oesophagus (Figure 2). A prospective randomised sham-controlled trial showed a significant improvement in symptoms in the APC arm. Long-term efficacy after a median follow-up of 17 months was shown in 76% of patients [4].
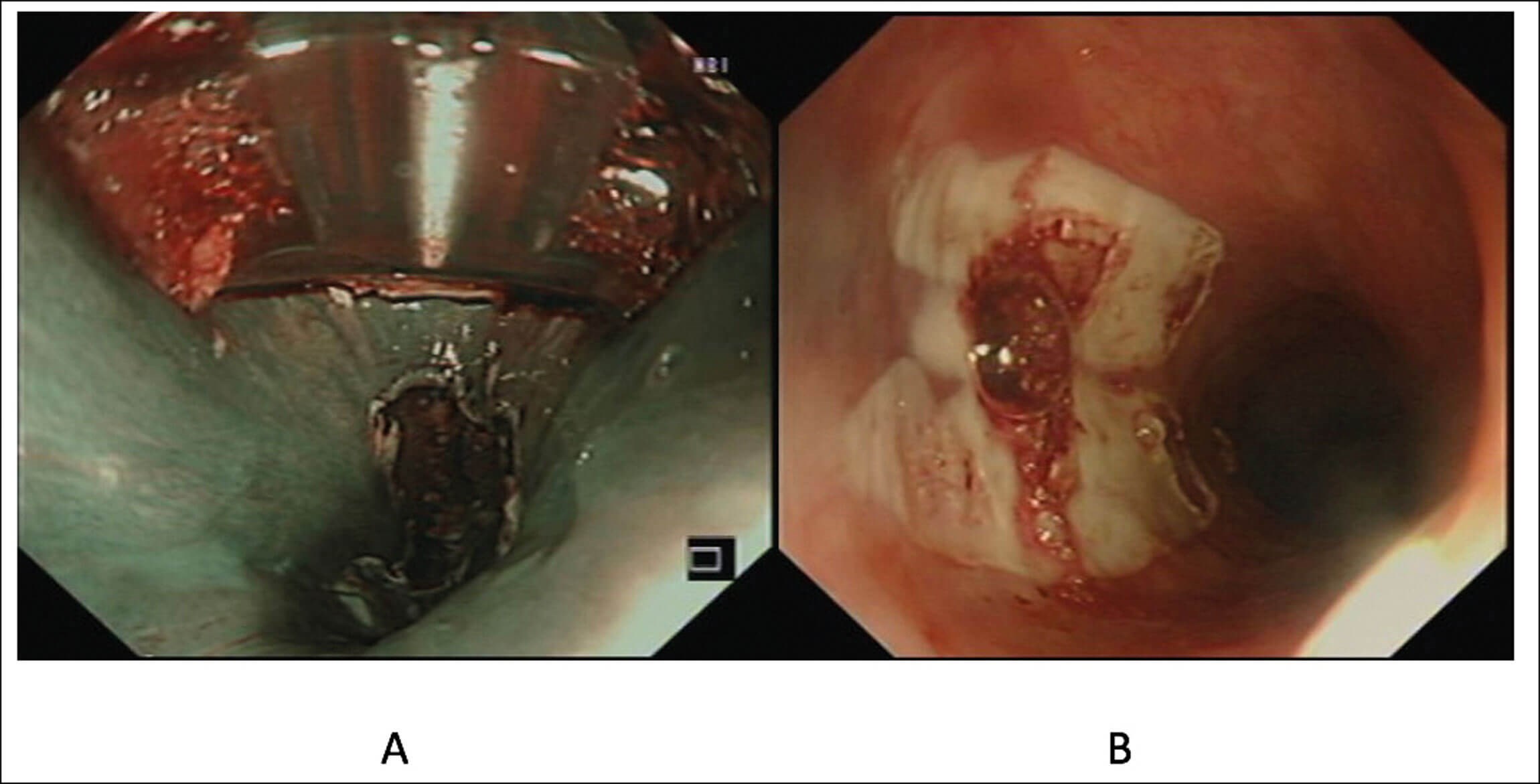
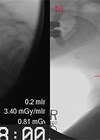
Figure 3. Radiofrequency ablation of an inlet patch performed under narrow
band imaging (A) and ablated area assessed after completion (B).
Radiofrequency ablation
RFA using a through-the-scope catheter is a novel technique for the management of GIP (Figure 3). A prospective pilot study of 10 patients with symptoms of sore throat and/or chronic globus showed that after a median of two treatment sessions with RFA, 80% achieved complete endoscopic and histological resolution of the GIP. Sore throat, globus and cough were significantly improved and durable for a median follow-up of 15 months. There were no complications [5]. There is currently an ongoing multicentre randomised sham-controlled trial lead by our group assessing the effectiveness of RFA in the treatment of an inlet patch by assessing for endoscopic resolution of the inlet patch as well symptom resolution.
Endoscopic resection
This is rarely performed in GIP. It may have a role with the use of endoscopic mucosal resection or endoscopic submucosal dissection in patients with evidence of dysplasia/early cancer in the inlet patch. This in itself is a rare occurrence.
"In benign inlet patches, ablative treatment with argon plasma coagulation (APC) or radiofrequency ablation (RFA) has shown to be beneficial"
Future perspectives
GIP is likely to be underdiagnosed and there is a growing area of evidence of its association with throat symptoms which can be disruptive to patients’ lives. A potential area for improvement is the inclusion of an image of the upper oesophagus as part of the quality indicators in endoscopy to ensure more GIPs are detected. Another area for improvement is the need for formal national guidelines for the management of this condition, which could potentially be a collaboration between gastroenterologists and ENT surgeons.
Outcomes from the RFA randomised control trial for the treatment of GIP will be published in due course and will potentially validate an important tool in the endoscopic armoury for the treatment of a gastric inlet patch.
References
1. Cock C, Hamarneh Z. Gastric inlet patches: symptomatic or silent? Curr Opin Otolaryngol Head Neck Surg 2019;27(6):453-62.
2. Rusu R, Ishaq S, Wong T, Dunn JM. Cervical inlet patch: new insights into diagnosis and endoscopic therapy. Frontline Gastroenterology 2018;9(3):214-20.
3. Neumann WL, Lujan GM, Genta RM. Gastric heterotopia in the proximal oesophagus (“inlet patch”): Association with adenocarcinoma arising in Barrett mucosa. Dig Liver Dis 2012;44(4):292-6.
4. Bajbouj M, Becker V, Eckel F, et al. Argon plasma coagulation of cervical heterotopic gastric mucosa as an alternative treatment for globus sensations. Gastroenterology 2009;137(2):440-4.
5. Dunn JM, Sui G, Anggiansah et al. Radiofrequency ablation of symptomatic cervical inlet patch using a through-the-scope device: a pilot study. Gastrointest Endosc 2016;84(6):1022-6.