Industry News
Soluvos Medical is looking forward to catching up with readers
Having recharged their batteries and the Soluvos Medical team is looking forward to meeting readers again at the next symposia and hands-on courses. Pass by their booth to catch up and to meet their new Sales Manager - Marc de...
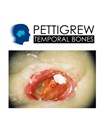
30 Years Experience with Pettigrew Temporal Bones
The Glasgow Temporal Bone Course was started in 1976, by Mr Alastair Pettigrew using cadaver bones. However, because of changes in the law in the UK, these became unavailable in the middle 90s and the course had to use the...
Join the leading event for tinnitus research and care – Tinnitus UK Conference 2025
Don’t miss the Tinnitus UK Conference 2025, the UK’s premier event dedicated to tinnitus research, clinical management and innovation. Taking place on 19 September 2025 in Birmingham and online, this one-day hybrid conference brings together leading researchers, audiologists, ENT specialists,...
MED-EL showcases global commitment to hearing health at World EXPO 2025 Osaka
At the heart of the Austrian Pavilion at World EXPO 2025, MED-EL is bringing hearing health to the global stage. Selected from over 150 applicants, MED-EL has been featured since April in Austria’s Innovation Lab, where an infomercial highlights how...
Soluvos Medical wishes readers a lovely summer season
Soluvos Medical had a busy conference schedule leading up to the summer break, and the team is now recharging and looking forward to meeting readers again at the next symposia and hands-on courses after the summer break. Find the Soluvos...
Neuxmed is now the exclusive UK and Ireland partner for Orlvision, a leader in German-designed and manufactured endoscopy systems
“At Neuxmed, our mission is to empower ENT specialists and speech and language therapists with innovative, reliable, and clinically proven endoscopic solutions,” says Michael Davies, MD at Neuxmed. “With over 12 years of experience in flexible endoscopic diagnostics, our team...
From melody to meaning: Meludia music training for CI users
Music and speech share deep cognitive and auditory roots. Meludia on myMED-EL, a music training especially curated for cochlear implant (CI) users, taps into this connection to support hearing performance. Meludia’s exercises can directly translate to better speech comprehension. -...
New tests enhance evaluation of dizzy patients
Interacoustics is pleased to announce an update to the popular VisualEyes™ VNG system which adds new data-driven tests designed to enhance the diagnostic evaluation and outcome of dizzy patients. VisualEyes 3.2 offers a new standalone package: VORTEQ™ Functional Assessments which...
One hundredth VOIS implant case completed
One hundred patients across the UK and Ireland have now benefited from an innovative vocal implant system designed to treat glottic insufficiency with voice and swallowing disorders. DP Medical Systems Ltd., the exclusive UK and Ireland distributor of the ‘VOIS...
Unlocking better reflux diagnostics with Peptest®
BIOHIT HealthCare recently hosted a fascinating webinar on Peptest, its innovative, non-invasive saliva-based test that is redefining reflux diagnosis. Led by Andrew Woodcock, Chief Scientific Officer at RD Biomed, the comprehensive session explored the science behind Peptest, from its early...
New innovative Head & Neck Ultrasound Course at St George’s Hospital London
The St George’s Emergency Head & Neck Ultrasound Course (18 September 2025) Emergency head and neck ultrasound is an increasingly vital diagnostic tool in acute care settings. It offers rapid, non-invasive assessment of critical structures including the airway, neck vessels,...
Nature Communications Medicine publishes positive, real-world data for tinnitus patients treated with unique, FDA approved stimulation device
Nature Communications Medicine has published the first peer reviewed, real-world analysis of U.S. patients treated with Lenire, the only FDA approved tinnitus treatment device of its kind. Results in the paper “Retrospective chart review demonstrating effectiveness of bimodal neuromodulation for...